X-ray Spectroscopy Helps Explain How FLASH Kills Cancer
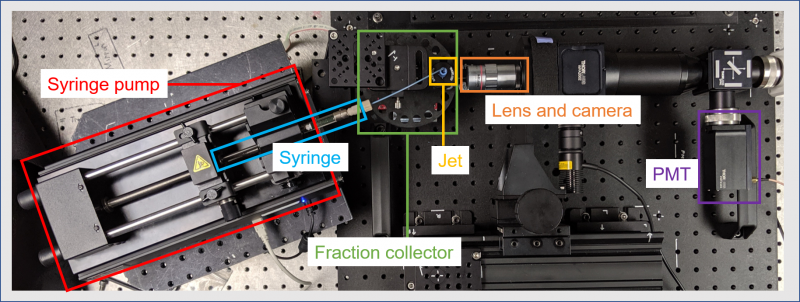 FLASH is a targeted radiation therapy that kills tumor cells while sparing healthy tissue. Like its namesake, FLASH delivers a short, intense burst of radiation in a single appointment. Despite this breakthrough’s proven ability, little is known about its mode of action against tumor cells. Lawrence Berkeley National Laboratory has been using X-ray footprinting mass spectrometry to investigate the mechanisms that make FLASH a powerful cancer killer.
FLASH is a targeted radiation therapy that kills tumor cells while sparing healthy tissue. Like its namesake, FLASH delivers a short, intense burst of radiation in a single appointment. Despite this breakthrough’s proven ability, little is known about its mode of action against tumor cells. Lawrence Berkeley National Laboratory has been using X-ray footprinting mass spectrometry to investigate the mechanisms that make FLASH a powerful cancer killer.
“FLASH refers to the phenomenon that very high dose rate irradiation will spare healthy tissue around a tumor, but still kill tumor cells to the same degree as conventional dose rate radiation,” said Corie Ralston, staff scientist and Facility Director, Biological Nanostructures. “The fact that this will spare healthy tissues is counterintuitive but has been demonstrated using different modes of radiation (X-ray, electron, proton) and in cells, tissues, and several animal models.”
First discovered in 2014, FLASH treatment can be significantly more potent than conventional treatment. There are many possible explanations as to why this more intense therapy works at the cellular level. One possibility is that high dose radiation produces extremely reactive ions and molecules that selectively damage cancer cells. Alternatively, the immune system might respond differently to the dosage level.
The research conducted by Ralston and her team points to a third theory – that a FLASH-induced low oxygen environment protects surrounding cells from further damage. At low oxygen levels, radiation induces fewer harmful modifications to proteins.
Using X-ray footprinting mass spectrometry to map specific protein modifications in cells under varying irradiation dose rates, the team found that oxygen is consumed quickly during treatment.
“We also found that high dose rate irradiation alters proteins less than low dose rate irradiation. This was counterintuitive, but matched the FLASH effect on healthy tissues,” Ralston said.
Both results support the “oxygen depletion effect” and lay the groundwork for future research using the X-ray footprinting method. With more mechanistic insights gained into FLASH, tailored dosage rates and treatment plans could be developed for each cancer type or patient.
“FLASH has generated huge interest in recent years and has been described as a breakthrough in radiation oncology,” said Ralston. “If the current clinical trials in humans hold up, then it might become the new standard of care for cancer treatment. It would mean that cancerous tumors could be treated faster and with far fewer side effects.”