Using an Auto-Planned VMAT-TBI Technique for Myeloablative Autologous Hematopoietic Stem Cell Transplantation for Scleroderma (The STAT-2 Trial)
Affiliations
- 1 Department of Radiation Oncology, Stanford University, Stanford, California
- 2 Department of Radiation Oncology, University of California, Irvine, Irvine, California
- 3 Department of Radiation Oncology, The University of Alabama at Birmingham, Birmingham, Alabama
- 4 Department of Medicine, Blood and Marrow Transplantation, Stanford University, Stanford, California
Objectives
The STAT-2 trial mandates lung and kidney sparing to 25% of the prescription dose and image guidance for kidney localization, posing challenges for institutions using conventional two-dimensional (2D) Total body irradiation (TBI) techniques. This study demonstrates implementation of an auto-planned volumetric modulated arc therapy-total body irradiation (VMAT-TBI) technique to facilitate STAT-2 patient enrollment and improve dissemination of modern TBI.
Materials/Methods
Our institution clinically implemented and automated VMAT-TBI treatment planning, and adapted scripts to meet STAT-2 trial requirements. Three patients were treated with 3-isocenter VMAT plans in head-first supine position and 2-isocenter anteroposterior and posteroanterior plans in feet-first supine position. A custom rotational platform facilitated patient orientation changes. Cone-Beam Computed Tomography provided image guidance for lung and kidney localization. Dosimetric indices for lungs and kidneys were retrospectively reviewed for three patients. Point doses were recorded at the head, neck, shoulder, mid-mediastinum, lumbar spine, hip, knee, and ankle to confirm dose uniformity.
Results
For a prescription dose of 8 Gy in 4 fractions, the average point doses for lungs and kidneys were 1.9±0.2 Gy and 1.9±0.4 Gy, respectively. Lungs_eval and kidney D mean were 2.6±0.1 Gy and 2.9±0.5 Gy, respectively. Eight anatomical dose points throughout the body met the prescription criteria within ±10% consistent with the trial constraint. The treatment was well tolerated with minor post-treatment toxicities (G1 diarrhea, G2 nausea, and G1 mucositis).
Conclusions
Average lung and kidney point dose constraints were achieved for the three patients. Dose–Volume Histogram metrics were achieved on average within 0.60 Gy for lungs_eval and 0.90 Gy for kidney volumes. VMAT-TBI offers superior treatment delivery for scleroderma patients, eliminating the need for heavy physical blocks and complexity of kidney localization. Auto-planning scripts are freely available on GitHub for wider VMAT-TBI adoption.
Introduction
Total body irradiation (TBI) is an important component in conditioning regimens for patients undergoing hematopoietic stem cell transplantation (HSCT). Depending on the type of transplantation, TBI may serve different purposes, such as suppressing the recipient’s immune system or killing the existing marrow cells to prevent graft rejection. However, the treatment-related risks of TBI can be significant, highlighting the need to develop techniques to mitigate treatment sequelae.
Modern radiation techniques to deliver TBI show promising advantages over current methods, including improvements in dose calculation accuracy, significant reductions in dose to organs at risk (OAR), and decreased patient toxicities.1 - 3 However, widespread implementation of these techniques has been hindered by the increased complexity of treatment planning and delivery. Conventional two-dimensional (2D) TBI involves placing the patient far from the radiation source at distances greater than 4 meters. The simplicity of planning and treatment for this technique has led to its dominance in TBI. However, multi-isocenter conformal arc therapy techniques such as volumetric modulated arc therapy (VMAT) and TomoTherapy provide attractive alternatives, especially when individualized OAR sparing must be prioritized.4
TBI is used to treat autoimmune diseases such as scleroderma, which is characterized by abnormally increased collagen synthesis and fibrosis that affects the skin, with variable involvement of the joints, lungs, heart, digestive tract, and kidneys. Conventional therapies involve immunosuppressive drugs; however, TBI followed by autologous HSCT has demonstrated significant clinical improvements in multiple trials.5 - 7 Despite these benefits, radiation therapy must be used with caution in these patients, owing to concerns for increased treatment-induced fibrosis.8 - 10 As a result, the TBI scleroderma trials mandated significant sparing of the lungs and kidneys to an upper limit of 2 Gray (Gy) for a prescription dose of 8 Gy (SCOT, STAT trials).11, 12 Challenges in meeting these constraints have been reported in the literature13 ; therefore, the purpose of this study was to evaluate the feasibility of adhering to them in the context of volumetric modulated arc therapy-Total body irradiation (VMAT-TBI).
Methods
Patient Cohort
This institutional review board-approved single-institution retrospective study focused on three patients who received VMAT-TBI from 2019 to 2023, who were also enrolled in the STAT-2 trial. Data collected from patient medical records and included demographics, disease characteristics, treatment details, outcomes, and follow-up.
VMAT-TBI Procedure
The VMAT-TBI technique used in this study has been described in detail in our previous publications,2, 14 - 16 and only a brief summary will be provided here. Full-body CT scans were acquired with a Siemens Biograph PET-CT scanner using 5 mm slice thickness. Patients were simulated on a custom rotational couch top (“Spinning Manny”) attached to the CT couch top. For these patients, two sets of plans were created owing to the limitations of the longitudinal travel extent of the treatment couch: VMAT plans with the patient positioned in the head-first supine position, and additional anteroposterior and posteroanterior (AP/PA) plans with the patient positioned in the feet-first supine position. The target was defined as the entire body contracted by 0.3 cm, subtracting lungs with a 0.5 cm isotropic margin and kidneys with a 0.5 cm margin medially; a 2.0 cm margin anteriorly/posteriorly and superiorly/inferiorly; and a 2.5 cm margin laterally. Plans were normalized such that the 90% planning target volume (PTV) body was covered by the prescription dose. Dosimetric planning objectives were based on STAT-2 recommendations, where lungs_eval and kidney volumes receive a mean dose (D mean ≤ 25% of the prescription dose), and the dose to the anatomical points is within 10% of the prescription dose. The anatomic points as defined by the trial were reference points distributed along the patient’s longitudinal axis (head, neck, shoulder, mid-mediastinum, lumbar spine, hip, knee, and ankle), as well as central points within the right lung and right kidney blocks. Each point was specified in the protocol as being located midway between the entrance and exit points of the opposed radiation beams of conventional TBI.
According to the STAT-2 trial recommendations, “If [lungs] shielding is done with MLCs, the above [lung block] edges shall be used for the lung contours, and optimization will be used to limit the mean lung dose to 200 cGy.” We interpreted this to mean that the lung_eval volume should receive less than 25% of the prescription dose. We followed the guidelines for lung blocks from the trial: “The lateral edges should be 1.0-1.5 cm from the inner border of the ribs, the inferior edges should be 1.0-1.5 cm from the dome of the apex of the diaphragm, 1.0-1.5 cm below the clavicles and the medial border, and 2.0-2.5 cm from the lateral edges of the thoracic vertebral bodies, with contouring to incorporate the hilae in the field.” Lung_eval volume was created using the block specifications above and was used for dosimetric evaluation of dose to lungs. From the trial text: “Right Lung (“Point 9”): This reference point is defined in the center of the right lung block. The point is taken to be midway between the entrance and exit points of the opposed radiation beams.” Accordingly, we evaluated the lung point dose at the mid-lung anterior-posterior separation and at the mid-lung superior-inferior extent. Similarly, the STAT-2 trial recommends kidney volume sparing to 25% of the prescription dose. In 2020, the planning process was automated due to the time-consuming nature of these cases, and the STAT trial volumes and constraints were incorporated into the automated scripts.14, 15
As presented in our previous works,14, 15 the auto-planning scripts automate many of the tedious and time-consuming tasks required for treatment planning in these cases; these include optimization structure, target, and plan creation, as well as isocenter/beam and isocenter placement. Furthermore, the optimization process was automated for performance of multiple successive optimizations without planner intervention. In addition, the developed software was made to be open source on GitHub to enable other clinics to adopt autoplanning into their own practice ( https://github.com/esimiele/VMAT-TBI-CSI ). All patient cases reported in this work were autoplanned using these scripts. Intensity-modulated radiation therapy quality assurance was performed using electronic portal imaging device portal dosimetry for each VMAT field with gamma criteria of 3%/2 mm with a 10% dose threshold. Gamma analysis, as first proposed by Low et al,17 is routinely used in radiation oncology to compare measured and calculated two-dimensional dose distributions that consider deviations in dose and distance domains where “gamma criteria” specify the maximum acceptable deviations in each domain. The magnitude of the deviation at every measurement point is calculated; if it falls within the ellipse created by the gamma criteria, the point is considered to pass (i.e., the deviation is acceptable), whereas a point outside of the ellipse fails. In addition, in vivo dosimetry using optically stimulated luminescence dosimeters was performed at the matchline between the VMAT and AP/PA portions of the patient’s treatment plan.
Toxicities
Toxicity data were identified by reviewing each patient’s weekly visit notes, their hospital admission notes during the peri-transplant period, and records from subsequent follow-up visits with the stem cell transplantation team. Acute toxicities were graded using Common Terminology Criteria for Adverse Events version 5.18
Results
Patient Characteristics
Three patients were identified and included in the analysis as shown in Table 1 . All three were female, and their ages at the time of radiation treatment were 34, 50, and 56 years. The median follow-up time was 46 months (range 20-64 months). The mean height and maximum width across patients were 162.1±4.5 cm and 49.7±5.3 cm, respectively.
Summary of the Dosimetric Evaluation of the Three Patients in the STAT-2 Trial Who Received VMAT-TBI Treatment
| Patient metrics | Min | Max | Average | σ |
|---|---|---|---|---|
| Patient height (cm) | 158.6 | 167.1 | 162.1 | 4.5 |
| Patient max width (cm) | 44.5 | 55.1 | 49.7 | 5.3 |
| Number of plan isocenters | 5 | 5 | 5 | 0 |
| PTV D90% (%) | 100 | 100 | 100 | 0 |
| PTV D1cc (%) | 123 | 128 | 126 | 3 |
| Lung R point dose (Gy) | 1.7 | 1.8 | 1.8 | 0.1 |
| Lung L point dose (Gy) | 1.7 | 2.4 | 2.1 | 0.3 |
| Lungs_eval D mean (Gy) | 2.6 | 2.7 | 2.6 | 0.1 |
| Kidney L point dose (Gy) | 1.5 | 2.3 | 1.9 | 0.4 |
| Kidney R point dose (Gy) | 1.6 | 2.4 | 1.9 | 0.4 |
| Kidneys D mean (Gy) | 2.4 | 3.4 | 2.9 | 0.2 |
Treatment Characteristics and Dosimetry
Treatment for each patient utilized five isocenters: head, chest, pelvis, upper legs, and lower legs. The dose prescribed was 8 Gy to be delivered in four fractions twice daily. In our cohort, the average mean dose was 2.6±0.1 Gy for the lung_eval (2.57 Gy, 2.74 Gy, and 2.90 Gy for each patient, respectively) and 2.9±0.5 Gy for the kidneys (3.36 Gy, 2.92 Gy, and 2.39 Gy for each patient, respectively). The average point dose measurements for the right and left lungs were 1.8±0.1 Gy and 2.1±0.3 Gy, respectively. For the right and left kidneys, the mean point dose measurements were 1.9±0.4 Gy each. The average plan D1cc (dose received by 1 cc of volume) was 126±3% ( Table 1 ).
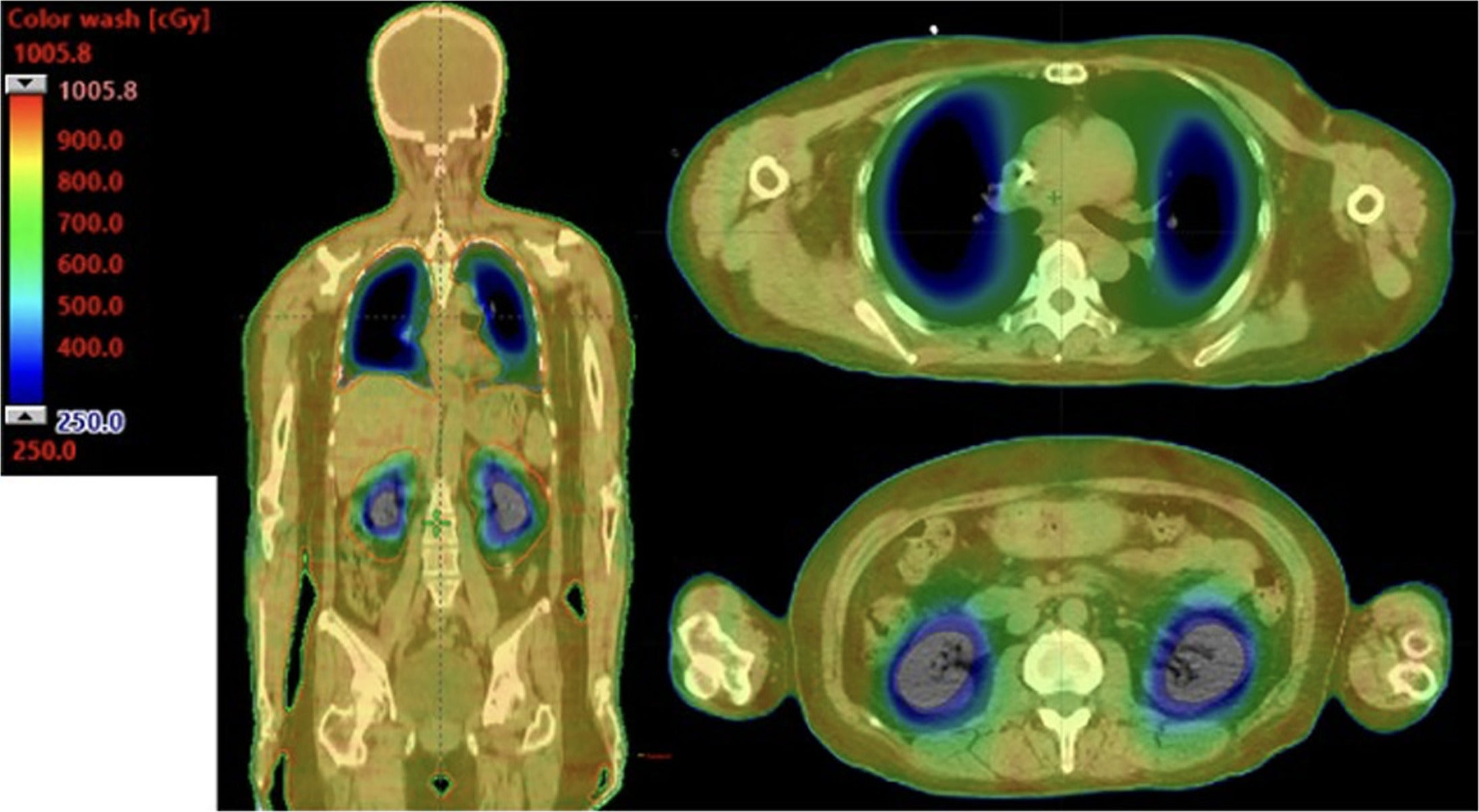
All three patient plans achieved 90% coverage of the PTV with 100% of the prescription dose. A sample plan dose distribution of a patient is shown in Figure 1 .
Coronal slices and axial slices from a patient demonstrating the dose distribution implemented in the STAT-2 trial using the VMAT-TBI technique. The visualization of the dose cloud is thresholded to 30% of the prescribed dose (2.5 Gy).
Toxicities
At the time of last follow-up (median 17.8 months, range 9.4-23.8 months), none of these patients experienced primary or secondary graft failure. There were no incidences of nephrotoxicity or pulmonary toxicity. Two patients experienced grade two toxicities (nausea), and no patient experienced any grade 3-5 toxicities.
Discussion
This single-institution report of three patients supports the feasibility of using VMAT-TBI to meet STAT-2 trial requirements. The STAT-2 trial mandates lung and kidney sparing to 25% of the prescription dose, and the average point dose values achieved for lungs and kidneys in our patients were 1.9±0.2 Gy and 1.9±0.4 Gy, respectively. Not only did VMAT-TBI eliminate the need for heavy physical blocks and circumvent the complexity of kidney localization associated with conventional 2D TBI treatment, but the treatment was also well tolerated with no incidences of grade 3+ toxicities, graft failure, or graft-versus-host disease.
Historically, investigators have reported a higher incidence of acute and/or late toxicities in cancer patients with autoimmune diseases receiving radiation therapy,8, 9, 19 - 24 leading to the cautionary use of radiation therapy in patients with scleroderma. A meta-analysis published in 2002 of 15 studies of patients with nonmalignant systemic diseases such as collagen vascular disease found high incidences of grade three or higher acute and late toxicities of 12.4-70% and 7-100%, respectively. The authors concluded that patients with collagen vascular disease have reduced radiation tolerance.19 However, the majority of recently published studies show no increased risk for acute or late toxicities in patients with collagen vascular disease,20, 25 which may be due in part to modern radiation treatment techniques. The recent CONTRAD meta-analysis of 18 studies, 10 of which included patients with collagen vascular disease, found a modest 10-15% risk of any grade 3+ toxicities, suggesting that collagen vascular disease is not an absolute contraindication to radiation therapy, as previously reported.22
A landmark randomized controlled trial by Sullivan et al reported improved overall survival with TBI compared to cyclophosphamide, albeit at the cost of increased toxicity to the kidneys and lungs. That study found treatment-related mortality in the transplant group of 3% at 54 months and 6% at 72 months, compared with 0% in the cyclophosphamide group.7 Collectively, the published data support the use of radiation therapy in cases of severe scleroderma, with the caveat of adopting a cautionary approach to minimize toxicities.
In contrast to the Sullivan et al trial, the SCOT, STAT, and STAT-2 trials mandate significant sparing of the lungs and kidneys with a dose restriction of ≤ 2 Gy for a prescription of 8 Gy.7, 11, 12 Although the strict kidney and lung dose criteria were formed to minimize radiation treatment toxicities in this patient population, recent studies have called into question the feasibility of achieving these constraints. Chiang et al performed a treatment planning study in which a validated 18 MV beam model was used to evaluate the resulting dose distribution from conventional AP/PA TBI with varying Cerrobend half-value layers (HVL).13 The SCOT protocol specifies block edges 1-1.5 cm from a lateral chest wall, clavicle, and diaphragm dome, and a 2-2.5 cm block margin from the lateral edge of the vertebral bodies, and for the blocks to be “2 HVLs thick” to achieve a lung dose of 2 Gy. Using these guidelines, the average central point dose under the lung block exceeded the mandated 2 Gy, and it was found that the 2 Gy lung dose could not be met, regardless of block thickness (owing to scatter from the blocks).
The requirement for kidney doses was more achievable, with three HVLs meeting a renal dose requirement of 2 Gy. However, the authors pointed out the impracticality of this approach, as three HVLs of kidney and lung blocks mounted on a plastic block tray can easily exceed 18 kg (40 lbs). In addition, Craciunescu et al highlighted the challenges in renal shielding mandated by the SCOT trial owing to the difficulty of localizing the kidneys in the standing position, and they describe methods to optimize renal shielding for conventional TBI techniques with a focus on plan robustness.26 Craciunescu et al measured average lung and kidney doses of 27.4% and 25.4%, respectively, based on extrapolated in vivo point dose measurements of 11 patients treated at their institution. However, as highlighted by Chiang et al, there can be significant differences between point dose measurements and mean organ doses depending on where the point dose is measured. Other studies have also noted discrepancies between measured, hand-calculated, and treatment-planning system-calculated doses for conventional TBI treatment techniques.27 - 29 Overall, these studies conclude there is considerable ambiguity in lung and kidney dose modulation for the 2D TBI techniques and recommend that future investigators develop more achievable, reproducible, and accurate TBI methodology.
In 2020, our institution clinically implemented multi-isocentric VMAT-TBI as an alternative to conventional TBI utilizing AP/PA beams. Although ongoing studies are investigating whether there are significant benefits of VMAT-TBI over conventional techniques, VMAT-TBI has improved dose calculation accuracy and the potential to overcome the impracticality of using multiple HVL Cerrobend blocks that achieve only sub-par dose sparing. Furthermore, VMAT-TBI has been shown to provide better OAR sparing,2, 14 - 16 offering a significant advantage for cases that require stringent dosimetry control levels. The reduced doses to OARs have translated to reduced toxicities. Hui et al reported a matched-pair single-institution retrospective analysis of 200 patients treated with TBI at our institution from 2014 to 2023.3 The VMAT-TBI cohort experienced significantly lower rates of any grade of pneumonitis (2% vs 12%), nephrotoxicity (7% vs 34%), nausea (68% vs 81%), skin (16% vs 35%), and graft-versus-host disease (42% vs 62%) compared to the 2D TBI cohort. For patients undergoing myeloablative regimens, rates of pneumonitis (0% vs 17%) and nephrotoxicity (9% vs 36%) were significantly lower with VMAT-TBI versus 2D-TBI. Similar outcomes were observed in the City of Hope study by Ladbury et al.1 Finally, Shinde et al reported pulmonary, renal, thyroid, and cataract toxicities from a prospective trial monitoring patients up to 8 years after TMI.30 Mean organ doses were lung 7.0 Gy, kidneys 7.1 Gy, thyroid 6.7 Gy, and lens 2.8 Gy. The crude incidence of radiation pneumonitis was 0.7% and no radiation-induced renal toxicity was noted.
These studies suggest VMAT-TBI offers improved organ sparing when compared to matched or historical cohorts treated with conventional TBI, which is paramount in this high-risk patient population. The key limitation of our study is the small number of patients. Thus, no meaningful conclusions should be drawn regarding the superiority of VMAT-TBI over conventional TBI techniques. The primary objective was to assess the feasibility of achieving the rigorous STAT-2 trial dose constraints for the kidneys and lungs using VMAT-TBI, which was successfully demonstrated in this study. Overall, this work demonstrates the feasibility of an automated solution for planning and treating patients on the STAT-2 trial. It also underscores the clinical relevance of VMAT-TBI by eliminating the need for Cerrobend blocks and their associated challenges; reducing toxicities by improving dose to OAR; enabling treatment for patients who cannot tolerate prolonged standing during conventional TBI; and providing open-source automated planning scripts that facilitate reproducibility and adoption across centers. Continued adaptation of our VMAT-TBI script to additional patients with the SCOT regimen will be necessary to validate our technique in this patient population and may lead to more explicit, reproducible, and accurate TBI methodology for future trials.
Conclusions
This study presents a promising advancement in TBI techniques with the potential to significantly influence patient treatment within the STAT-2 trial. The VMAT-TBI technique offers image guidance for accurate treatment delivery, uses MLCs for lung and kidney blocking that disposes of heavy Cerrobend blocks, and expands treatment to patients who cannot tolerate standing for the prolonged duration of conventional TBI treatment. The patients in this series have experienced mild toxicities with the automated VMAT-TBI method, underscoring its effectiveness and tolerability.