Treatment of Stage IIB Seminoma in a Patient With Down Syndrome and Eisenmenger Syndrome: A Case Report
Affiliations
- 1 Radiation Therapy Department, Unidade Local de Saúde de São João, Porto, Portugal
- 2 Urology Department, Unidade Local de Saúde de São João, Porto, Portugal
Background
Management of testicular germ cell tumors in patients with complex comorbidities remains challenging. We present a case of stage IIB seminoma in a patient with Down syndrome (DS) and Eisenmenger syndrome (ES).
Case Presentation
A 37-year-old man with DS and ES underwent radical orchiectomy for a testicular mass, confirming seminoma (pT2N2M0R0S0). Following disease progression during surveillance, external beam radiation therapy (36 Gy in 18 fractions) was administered using a dog-leg field technique, as both chemotherapy and surgery were contraindicated. Treatment was well-tolerated with only mild nausea. Follow-up imaging showed near-complete response at 4 months, with stable disease at 12-month follow-up.
Conclusion
This case demonstrates radiation therapy as an effective, well-tolerated treatment for stage IIB seminoma. Despite theoretical concerns regarding radiosensitivity in DS and the hemodynamic risks of thoracic irradiation in ES, standard para-aortic/iliac radiation was delivered safely, achieving disease control without unexpected toxicity.
Introduction
Testicular germ cell tumors (TGCTs) represent 1% to 2% of all cancers in men and are the most common solid tumors found in male adolescents and young adults.1 Most testicular tumors (95%) arise from germ cells and can be divided into seminomas and non-seminomas, each with distinct biological behaviors and treatment responses.2, 3
Treatment approaches for TGCTs vary according to histological subtype and disease stage. For stage IIB seminomas (characterized by metastasis with a lymph node mass between 2 and 5 cm in greatest dimension; or >5 nodes, positive, and none larger than 5 cm; or evidence of extranodal extension of the tumor), several treatment options exist, including radiation therapy (RT), chemotherapy (CT), and retroperitoneal lymph node dissection (RPLND).4 - 6 Historically, RT has maintained a crucial role in the treatment of these tumors, with stage IIA seminoma typically treated with 30 Gy to the para-aortic and ipsilateral iliac lymph nodes and stage IIB seminoma treated with an escalated dose of 36 Gy.7
Multiagent CT regimens, particularly bleomycin, etoposide, and cisplatin or etoposide and cisplatin, have become the preferred first-line approach to treating most stage II seminomas due to their efficacy and, potentially, lower long-term toxicity profiles. However, there are limited data on the outcomes of these regimens in patients with significant comorbidities or genetic syndromes, representing a substantial knowledge gap in the literature.8
Down syndrome (DS), characterized by trisomy 21, presents a unique cancer predisposition profile, where the incidence of testicular cancer is 5-fold that of the general population.9 - 12 In addition, evidence has suggested a radiosensitive cellular phenotype in DS, linked to superoxide dismutase (SOD1) overexpression and defective DNA synthesis checkpoints after γ-irradiation.13, 14 However, clinical studies have not demonstrated excess RT-related toxicity in this population.15
Eisenmenger syndrome (ES) is particularly prevalent in individuals with DS.16 The condition limits cardiopulmonary reserve in these patients, significantly constraining oncological management options.17 - 19 In this context, field selection is critical: para-aortic and iliac RT fields largely spare the heart and lungs, thereby minimizing hemodynamic stress, whereas thoracic fields may exacerbate pulmonary hypertension and right heart strain. For such patients, RT may offer advantages by avoiding systemic toxicities, though the patient’s cardiac and pulmonary function must be carefully factored into treatment planning.20, 21
The aim of this case report is to highlight the management of stage IIB seminoma in a patient with DS and ES, while demonstrating the value of RT as a treatment modality in this unique clinical scenario.
Case Presentation
A 37-year-old patient with a medical history of DS and ES presented for treatment with an enlargement of the left testicle, noticed by his parents. The patient had undergone previous surgery for a sacrococcygeal fistula and had no history of smoking or alcohol consumption. There were no relevant chronic medications, no known drug allergies, and no family history of oncological diseases.
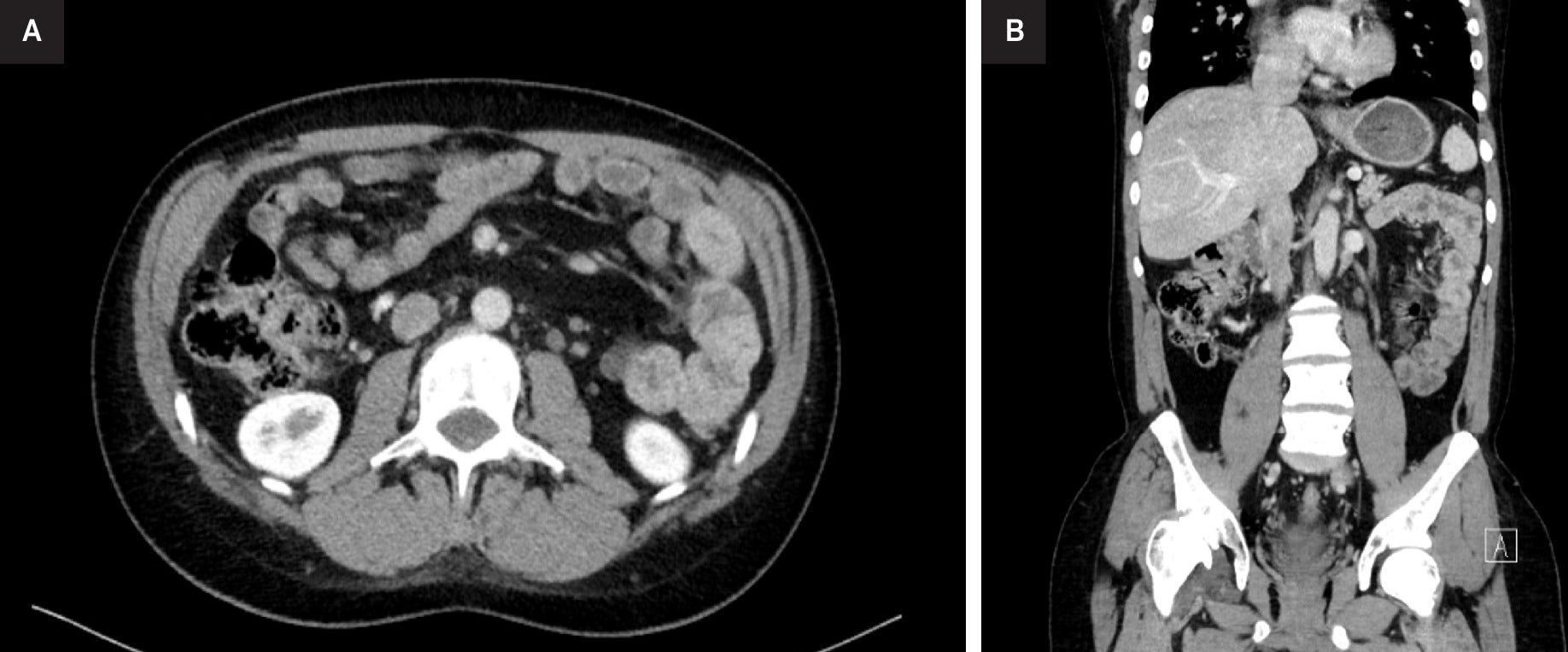
The patient was comfortable at rest, without any pain, fever, weight loss, or other symptoms. Examination revealed a mass on his left testicle without inguinal or other palpable adenomegalies. A scrotal ultrasonography revealed a well-defined left testicular solid tumor, with heterogeneous echogenicity. Following admission, routine blood work showed a mildly elevated white cell count at 12.4 × 109 /L and a β-human chorionic gonadotrophin slightly elevated at 5.98 mIU/mL. All other blood markers, including tumor marker levels of α-fetoprotein and lactic dehydrogenase, were within normal range. A thoraco-abdominal-pelvic tomography (CT-TAP), performed in the same month, revealed a retroperitoneal left para-aortic lymphadenopathy, located below the renal vein, measuring 20 mm, suggestive of metastatic spread. A large heterogeneous expansive lesion was identified ( Figure 1 ).
Thoraco-abdominal-pelvic CT. axial (left, A) and coronal (right, B) images demonstrate a retroperitoneal left para-aortic lymphadenopathy, located below the renal vein, measuring 20 mm.

The patient, through family representatives, declined both sperm cryopreservation and testicular prosthesis placement. A left inguinal orchiectomy was performed. The postoperative course was complicated by scrotal hematoma formation. The histopathological exam confirmed a left testicular seminoma, categorizing the tumor into pT2N2M0R0S0 with invasion of rete testis and >4 cm.22 Given the patient’s complex clinical history with significant comorbidities, the Urology Multidisciplinary Tumor Board recommended active surveillance rather than adjuvant therapy.
During the surveillance period, a follow-up CT-TAP at 4 months revealed, of oncological significance, a retroperitoneal lateroaortic lymphadenopathy, with the largest node measuring 23 × 22 mm, suspicious for nodal metastasis.
Given these findings, particularly the enlarging retroperitoneal lymphadenopathy consistent with stage IIB seminoma, the patient was again presented to the Urology Tumor Board, which recommended external beam RT with curative intent, specifically dog-leg field RT. Due to his comorbidities, the patient was deemed unsuitable for CT.
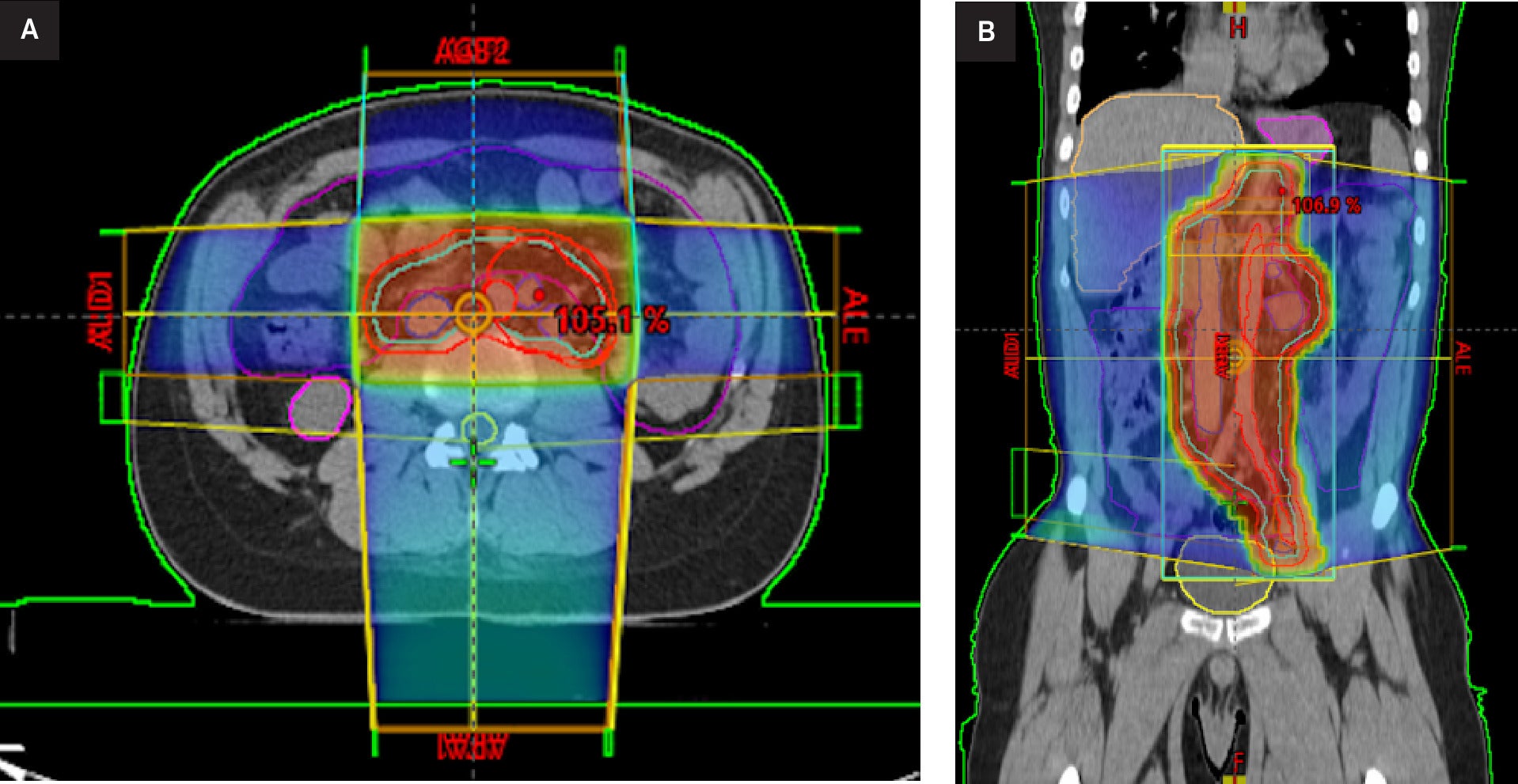
The patient subsequently underwent RT treatment with a 3 DCRT dog-leg technique, receiving a total dose of 20 Gy to the lumboaortic and iliac regions followed by an additional dose (boost) of 16 Gy to the left lateroaortic mass, 2 Gy per fraction, with a total dose of 36 Gy in 18 fractions, once daily ( Figure 2 ). Thoracic organs were outside the field; reported metrics were therefore negligible (mean ≈ 0 Gy, V5 ≈ 0%), eliminating cardiopulmonary exposure. Measures were taken to avoid epileptic spasms, including continuous pulse oximetry, avoidance of hypoxia/hypercarbia, and cardiology oversight. No anesthesia or sedation was required.
External beam radiation therapy treatment planning. Axial (A) and coronal (B) CT images with overlaid dose distribution and target volume (green outline).
The patient tolerated treatment well, experiencing only mild nausea, with no other documented symptoms. A reassessment CT-TAP scan after 3 months revealed a reduction in left lateral aortic adenopathy to 13 × 10 mm, with the disappearance of the remaining metastatic lymph nodes.
Since undergoing RT, the patient has remained in good health, asymptomatic, and free of treatment-related toxicities. Follow-up imaging at 1 year revealed a complete response (CR) regarding the left lateroaortic adenopathy according to RECIST criteria ( Figure 3 ).23 The patient remains stable at 12-month follow-up.
Reassessment thoraco-abdominal CT with 3-dimensional reconstruction at 3 m. Axial (A) and coronal (B) views demonstrate reduction in left lateral aortic adenopathy (now measuring 13 × 10 mm) with disappearance of previously identified metastatic lymph nodes.
Discussion
This case report demonstrates the successful management of stage IIB seminoma in a patient with DS and ES using adjuvant RT. The CR achieved with minimal toxicity highlights RT as an effective option in this complex clinical scenario.
The most relevant finding of our case is that standard RT protocols can safely and effectively treat stage IIB seminoma in patients with DS and ES. The patient’s excellent response—significant nodal reduction at 1 month and CR per RECIST criteria at subsequent follow-up—reinforces the high radiosensitivity of seminoma even in patients with complex comorbidities. This outcome is consistent with established data showing 5-year relapse-free survival rates of 90% for stage IIB seminoma treated with RT.24
The favorable toxicity profile observed in this case is particularly noteworthy given long-standing theoretical concerns regarding altered tissue radiosensitivity in individuals with DS.25 Historically, increased radiosensitivity in DS has been postulated based on early in vitro findings showing reduced survival of DS fibroblasts after irradiation, potentially linked to overexpression of Cu/Zn-SOD1, encoded on chromosome 21.13 Additional studies have reported abnormal DNA synthesis checkpoints following γ-irradiation in DS cell lines, suggesting an impaired DNA damage response.14 Although these biological observations imply a predisposition to heightened radiation sensitivity, clinical data remain inconsistent, and no definitive contraindication to standard RT regimens has been established. In the present case, the absence of significant acute or subacute toxicities following a total dose of 36 Gy further supports that contemporary RT techniques and conventional fractionation can be administered safely in DS patients with appropriate monitoring.
Our approach aligns with historical practice in which RT was the primary treatment for stage IIA/B seminoma, with recommended doses of 36 Gy for stage IIB.7 However, it diverges from contemporary trends that favor multiagent CT regimens as first-line treatment.26, 27 This decision was justified by the patient’s unique clinical context, where bleomycin-containing regimens posed substantial pulmonary toxicity risks.19 The anatomic distribution of stage IIB seminoma allowed the use of dog-leg fields confined to the para-aortic and ipsilateral iliac regions. This strategy completely avoided lung and heart irradiation, eliminating additional cardiopulmonary burden—a critical factor in patients with ES.
While recent literature has explored de-escalation approaches, such as carboplatin combined with involved-node RT as in the SAKK 01/10 trial, these approaches lack sufficient evidence for routine clinical use, particularly in patients with complex comorbidities.28 Similarly, although RPLND has emerged as an alternative with 2-year recurrence rates of 18%, the substantial perioperative risks associated with ES made this approach prohibitively dangerous for our patient.5, 29
Our case addresses a critical knowledge gap regarding testicular cancer management in patients with DS and cardiovascular complications. Patients with DS paradoxically show 5-fold higher rates of TGCTs despite lower solid malignancy rates, suggesting specific genetic, developmental, or hormonal factors possibly linked to higher cryptorchidism prevalence.30, 31 The successful outcome in this case demonstrates that RT can serve as an effective therapeutic modality, providing an important treatment option for similar patients where the standard approaches might pose unacceptable risks.
The initial decision to pursue active surveillance followed by prompt RT upon disease progression exemplifies the value of tailored approaches based on individual risk factors and disease characteristics. The integration of urological, radiation oncology, medical oncology, and cardiology expertise was essential in formulating an optimal treatment plan, highlighting the importance of multidisciplinary tumor boards in managing complex oncological cases. Despite theoretical concerns about RT in patients with DS, our case suggests that standard dose-fractionation schedules can be safely administered with appropriate monitoring and supportive care.
This report strengthens the limited clinical evidence suggesting that theoretical radiosensitivity in DS does not preclude the safe use of standard RT regimens. When delivered with precise planning that avoids thoracic exposure, RT may represent the safest and most effective curative modality in patients with seminoma and ES.
As a single case, this report cannot establish definitive recommendations, and longer follow-up is required to assess late effects and secondary malignancies. Nevertheless, it highlights a scenario where RT provided excellent disease control with negligible toxicity in a patient population that often falls outside the evidence base of clinical trials. Further research is warranted to elucidate characterization of testicular cancer in DS, evaluate different treatment modalities, and develop tailored surveillance protocols for this high-risk population.
In conclusion, RT can represent a safe and effective treatment modality for stage IIB seminoma in patients with DS and significant cardiovascular comorbidities such as ES. This approach offered excellent disease control while avoiding the potential complications associated with systemic therapy or surgical management in this medically complex patient.