The Effect of Pentoxifylline and Vitamin E in Preventing Grade 3 Radiation Pneumonitis: A Single-Arm, Phase II Prospective Study
Affiliations
1 Department of Radiation Oncology, Brown Cancer Center, University of Louisville School of Medicine, Louisville, KY
2 Department of Bioinformatics and Biostatistics, University of Louisville, Louisville, KY
Objective/Hypothesis
Stereotactic ablative radiation therapy (SABR) is becoming an increasingly popular treatment for patients with recurrent non-small cell lung cancer. Thoracic reirradiation, however, can be toxic, with some institutions reporting grade 3 pneumonitis in upward of 30% of reirradiated patients. Pentoxifylline (PTX) and vitamin E (VE) have mitigated toxicity in standard breast treatment and may be beneficial in the reduction of radiation-induced pneumonitis. The objective of this study is to prospectively evaluate the efficacy of PTX and VE in reducing grade 3 pneumonitis in patients undergoing SABR with locoregionally recurrent lung cancer or new lung primary tumors in the setting of prior thoracic radiation. We hypothesize that these patients will experience rates of grade 3 pneumonitis lower than 30% at 3, 6, and 12 months post-treatment.
Materials and Methods
Patients who received radiation for a prior thoracic malignancy with a diagnosis of a recurrent or new NSCLC were recruited from our institution. PTX and VE were administered at the time of simulation, approximately 1 week prior to starting treatment, and were continued for 12 weeks post-treatment. SABR was delivered using standard stereotactic techniques to a dose of 50 Gy at 10 Gy per fraction over 2 weeks. Clinical and radiographic assessment of pneumonitis was conducted at 3, 6, and 12 months post-treatment. Demographic information was collected before treatment.
Results
The rate of grade 3 pneumonitis in our PTX- and VE-treated cohort was significantly lower than 30% at 3 months (0%, 95% CI 0%-11%, P = .001), 6 months (5%, 95% CI 0%-20%, P = .004), and 12 months (0%, 95% CI 0%-21%, P = .010) post-treatment. Also, 92% of participants were medication compliant.
Conclusion
PTX and VE are safe interventions that may reduce rates of grade 3 pneumonitis for patients undergoing reirradiation for locoregionally recurrent and/or new lung primary tumors.
Keywords: non-small cell lung cancer, stereotactic ablative radiation therapy, vitamin E, pentoxifylline, reirradiation, pneumonitis
Introduction
Stereotactic ablative radiation therapy (SABR) is a versatile treatment for patients with non-small cell lung cancer (NSCLC) in the settings of operable early stage disease and inoperable late-stage disease.1 Despite advancements in this radiotherapeutic technique, recurrence is not uncommon, with studies suggesting in-field failure rates of up to 30% at 2 years post-treatment, particularly in the setting of concurrent chemoradiation for stage 3 NSCLC.2 Traditionally, systemic therapy was the choice approach for instances of loco-recurrent relapse; however, poor response rates prompted investigations regarding the safety and efficacy of reirradiation.
In addition to high rates of local failure, patients undergoing reirradiation with fractionated external beam radiation therapy have also experienced toxicities such as esophagitis, dry desquamation, and symptomatic pneumonitis, arguing for the need to dose-escalate using conventionally fractionated regimens.3 Institutional data evaluating SABR to treat locoregional lung cancer recurrences and new lung primaries in patients who have received prior thoracic radiation therapy is promising, with in-field local tumor control >90% at 2 years and 2-year overall survival (OS) and progression-free survival of 59% and 26%, respectively.4 Unfortunately, rates of grade 3 toxicities were 30%.4 A more recent review article by the International Association for the Study of Lung Cancer Advanced Radiation Technology Committee agrees that SABR is efficacious, with rates of 2-year local control ranging from 75% to 100%, despite persistence of toxicities.5
Prior studies have attempted to mitigate the effects of radiation on normal lung by using radioprotective agents such as pentoxifylline (PTX).6 - 8 PTX is thought to help prevent radiotoxicity by inhibiting platelet aggregation and adhesion, allowing for optimal blood flow and increased tissue oxygenation.9 Adding PTX to the retreatment of thoracic malignancies may aid in further reducing radiation-induced effects on normal adjacent tissue. A recent randomized study in breast cancer demonstrated that PTX in combination with vitamin E (VE) resulted in reduced rates of breast fibrosis after radiation therapy.10 A randomized controlled trial evaluating VE and PTX in primary lung radiation showed increased rates of radiation-induced lung toxicity in the control group.11 However, to date, no studies have assessed the efficacy of PTX and VE in the prevention of toxicities in the setting of reirradiation using SABR for lung malignancies.
Our phase II prospective trial addresses this gap in the literature by (1) evaluating the use of SABR in treating patients with locoregionally recurrent lung cancer or new lung primary tumors in the setting of prior thoracic radiation therapy and (2) establishing the efficacy of PTX in combination with VE in reducing the rates of grade 3 or 4 toxicities. Each patient was treated with VE and PTX before, during, and after reirradiation for lung malignancy via SABR. We hypothesize that SABR will be a viable treatment modality for these patients and that PTX and VE will reduce the rate of grade 3 pneumonitis in the cohort.
Materials and Methods
Patients were enrolled in a prospective, single-arm trial at our institution to assess the effects of PTX and VE on the rates of toxicity while using SABR in patients with recurrent or new lung primary after receiving prior thoracic radiation therapy. The criterion for patient eligibility consisted of previous radiation therapy for a thoracic malignancy with a diagnosis of a recurrent or new NSCLC. Patients either underwent a biopsy to confirm the diagnosis or demonstrated a strong clinical suspicion for new or recurrent cancer based on the recommendations of a multidisciplinary thoracic oncology team. Patients were excluded for the following reasons: <30 Gy of overlap from prior radiation treatment, poor pulmonary function at baseline (FEV1 < 20% and/or diffusing capacity of the lungs for carbon dioxide <20% predicted), chemotherapy within 4 weeks of SABR initiation, and a plan to initiate chemotherapy or immunotherapy concurrent with SABR.
Patients were administered PTX 400 mg 3 times daily. This was the standard starting dose for the drug as recommended by the manufacturer. Patients were given VE 400 IU once daily. Dosing began 1 week prior to treatment, approximately at the time of simulation. The drugs were continued for a period of 3 months after completion of radiation therapy. Dose delays or modifications were based on toxicity and made at the discretion of the principal investigator.
All patients were prescribed 50 Gy delivered to the planned target volume (PTV) in 5 fractions at 10 Gy per fraction. Fractions were administered at least 36 hours apart, and therapy was completed within 14 days of initiation. Daily image guidance with cone beam CT was required. Respiratory gating or abdominal compression was utilized as deemed appropriate by the treating physician and physicist.
Treatment planning was performed using either 3-dimensional conformal therapy or intensity- modulated radiation therapy. Any combination of coplanar or noncoplanar fields designed to cover the target volumes while limiting dose to critical structures was allowed. If prior dosimetry was available, a composite of the dosimetry plans of the prior treatment volume and the new treatment plan was generated. Standard SBRT treatment planning was utilized. Successful treatment planning was designed to meet the following:
-
Normalization: The plan was normalized such that 100% of the dose delivered corresponded to the center of mass of the PTV.
-
Prescription isodose surface coverage: The prescription isodose surface was chosen such that 95% of the PTV was conformally covered by the prescription isodose surface, and 99% of the PTV received at least 90% of the dose.
-
High-dose spillage: Any dose >105% of the prescription isodose surface occurred primarily within the PTV itself and not within normal tissue. The cumulative volume of all tissue outside the target received no more than 105% of the prescription to 15% of the volume. PTV conformality was judged such that the ratio of the volume of the prescription isodose to the volume of the PTV was ideally <1.2 as outlined by the Radiation Therapy Oncology Group.
-
Standard dose constraints to critical structures: Outlined in accordance with the Quantitative Analyses of Normal Tissue Effects in the Clinic.
The Common Terminology Criteria for Adverse Events (v. 4.0) was used to assess radiation- and PTX-related toxicity. Standard radiation-related toxicities were expected. Toxicity was defined as acute (<3 mo from completion), subacute (3-12 mo), and late (>12 mo). Standard consent forms for lung irradiation were used for informed consent. Specifically, radiation pneumonitis and fibrosis were assessed. The CT appearance of the consolidation was classified as either diffuse or mass-like according to published criteria.12 Diffuse consolidation was defined as consolidation occurring outside of the 50% isodose line. Mass-like consolidation was defined as a new or enlarging solid opacity occurring within or directly adjacent to the PTV.
Standard post-treatment follow-up occurred at 6 weeks and then every 3 months after the completion of radiation therapy. A complete history and physical examination were performed at each follow-up visit to assess for treatment-related toxicity. Pulmonary function tests with lung diffusion testing were ideally obtained at 6 months post-radiation and yearly as determined by the treating physician. Follow-up imaging took place 8-12 weeks post-radiation and then every 3 months thereafter for 2 years. CT of the chest was performed using intravenous contrast. Surveillance CT imaging of the chest was obtained as recommended by the treating physician. F-18 fluorodeoxyglucose-positron emission tomography was obtained at 6 months post-SABR and then when determined clinically relevant by the treating physician.
Statistical Design
The primary endpoint was to estimate overall treatment-related toxicity and assess the efficacy of PTX in reducing grade 3 or 4 toxicity. The study was designed to estimate grade 3 treatment-related toxicity. Reports from prior retreatment series estimate >grade 3 pulmonary toxicities to be approximately 30%.4 Our goal was to reduce this rate to 15%.
The cumulative number of grade 3 or 4 toxic events was monitored after each patient was enrolled. If the cumulative number of toxic events produced enough evidence to conclude that the true toxicity rate is ≥30% (Pt0 = 0.30), then the trial was planned to stop early for safety reasons.
Descriptive statistics were used to summarize the demographic and clinical features of the cohort. Toxicity was analyzed by taking the highest/most severe score within the first 3 months post-treatment, between 3 and 6 months post-treatment, and between 6 and 12 months post-treatment. At each point, the proportions for each toxicity score were computed, and the primary analysis was based on a one-sided test that the rate of grade 3+ toxicity is <30%. To assess the relationship between the ordinal toxicity scores and demographic and clinical characteristics, we considered proportional odds regression. We included time (3 mo, 6 mo, 12 mo) as a predictor in all models and otherwise included predictors one at a time. As we used multiple scores from each individual, we included a random intercept term to introduce correlation between the repeated measures.13 Odds ratios were rescaled using the random effect variance to provide an approximately marginal interpretation.14 Kaplan-Meier curves of OS and for recurrence-free survival (RFS) were considered. Missing data were addressed by performing an available case analysis. Statistical significance was defined at the alpha = 0.05 level, and all data analysis was performed using R statistical software, v.4.3.3.15
Of note, noncompliant patients were included in all analyses for which they had available data to contribute. One noncompliant patient died before post-treatment data were collected and was not included in our results; 2 other noncompliant patients contributed toxicity and survival data at each timepoint.
Results
Demographics
Demographic information about our cohort of 40 patients, all recruited from our institution, is displayed in Table 1 . Of those patients, 98% had a baseline Eastern Cooperative Oncology Group (ECOG) status of ≤1. The average previous radiation dose prior to enrollment was 56.9 Gy; individual prior radiation doses and fractionations are listed in Table 2 . 93% of participants were able to receive the anticipated reirradiation dose of 50 Gy. Overall, PTX and VE were well tolerated, with only 3 patients reporting noncompliance during the study.
Participant Demographics
| Column1 | Mean/Count | Standard Deviation | Range | Missing |
|---|---|---|---|---|
| Patients | 40 | |||
| ECOG | 0 | |||
| 0 | 15 | 38% | ||
| 1 | 24 | 60% | ||
| 2 | 1 | 3% | ||
| Previous radiation dose (Gy) | 56.9 | 10.8 | 30-73.5 | 6 |
| Interval between doses (mo) | Median 12 | IQR 12–27 | 8-120 | 4 |
| >12 mo | 17 | 47% | 4 | |
| Dose at retreatment (Gy) | 0 | |||
| 50 | 37 | 93% | ||
| 25 | 1 | 3% | ||
| 40 | 2 | 5% | ||
| Central tumor (vs peripheral) | 6 | 16% | 3 | |
| Noncompliant | 3 | 8% | 0 | |
| Pretreatment FEV1 | 1.52 | 0.65 | 0.78-3.66 | 20 |
Eastern Cooperative Oncology Group (ECOG) performance status scale, patients scoring 0 are fully independent, patients scoring 1 are restricted in strenuous activity but able to perform basic ADLS, patients scoring 2 are ambulatory however limited in daily work activities.
Forced expiratory lung volumes (FEV1) as measured on pulmonary function tests (PFTs).
Individual Prior Radiation Doses and Fractionation
| Number of Patients | Previous Radiation Dose (Gy) | Previous Fractions | Previous Biological Equivalent Dose |
|---|---|---|---|
| 1 | 30 | 10 | 39 |
| 1 | 33 | 10 | 43.8 |
| 1 | 37.5 | 15 | 46.8 |
| 1 | 45 | 15 | 58.5 |
| 1 | 45 | 25 | 53.1 |
| 3 | 48 | 4 | 105 |
| 1 | 50 | 5 | 100 |
| 2 | 50.4 | 28 | 59 |
| 3 | 54 | 3 | 151 |
| 2 | 59.4 | 33 | 70 |
| 5 | 60 | 30 | 72 |
| 1 | 61.2 | 30 | 71 |
| 1 | 63 | 30 | 73 |
| 1 | 65 | 33 | 71 |
| 4 | 66 | 33 | 72 |
| 1 | 66.4 | 35 | 74 |
| 1 | 66.4 | 33 | 72 |
| 1 | 66.6 | 33 | 72 |
| 1 | 68.4 | 38 | 80 |
| 1 | 73.5 | 35 | 74 |
Grades of Radiation Pneumonitis and Drug-Related Toxicities
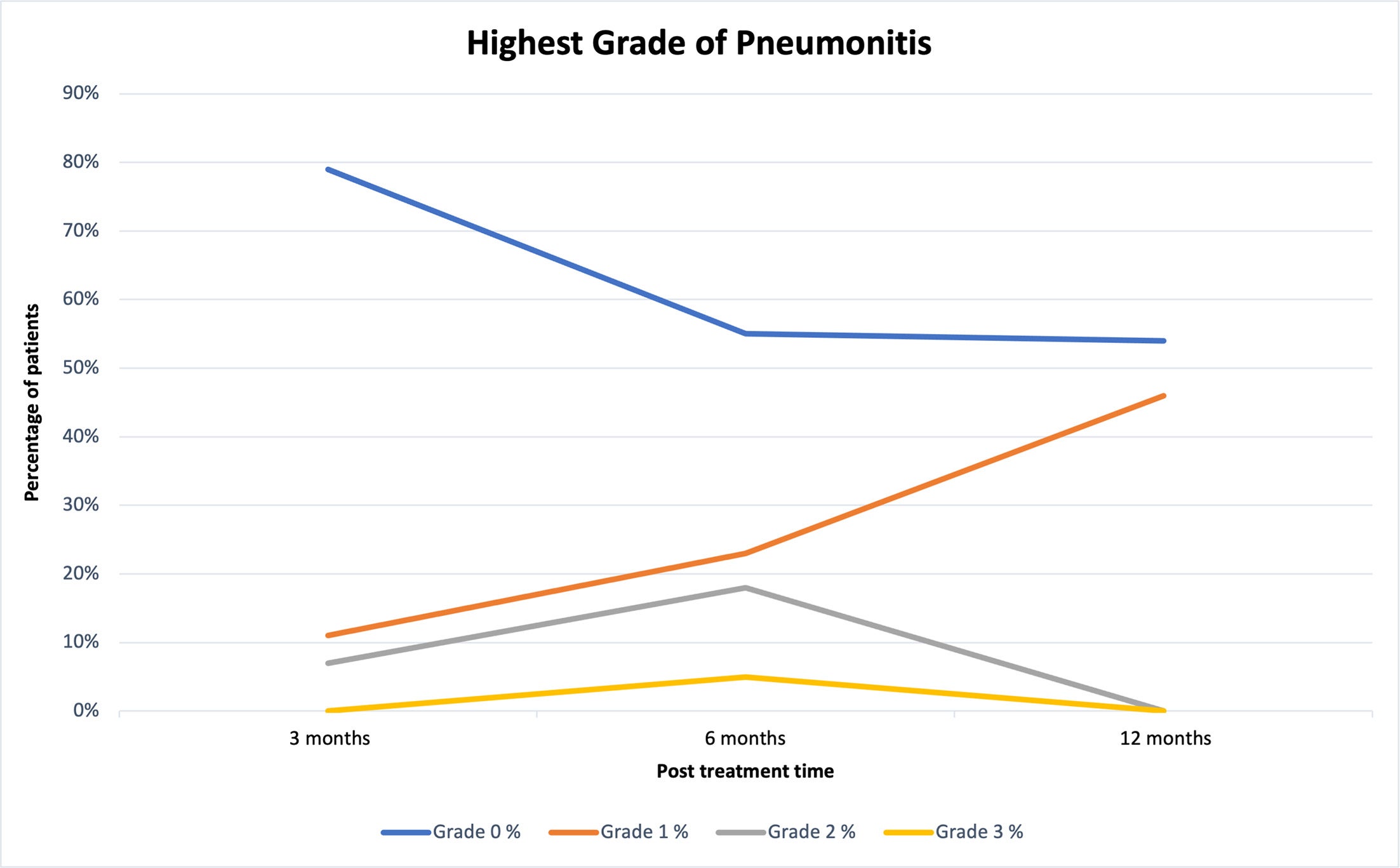
Toxicity results are outlined in Table 3 and graphically displayed in Figure 1 . Of note, only one patient experienced grade 3 pneumonitis, which occurred at 6 months post-treatment. Furthermore, there is statistically significant evidence that rates of grade 3 pneumonitis are less than 30% at 3 months (0%, 95% CI 0%-11%, P = .001), 6 months (5%, 95% CI 0%-20%, P =.004), and 12 months (0%, 95% CI 0%-21%, P = .010) post-reirradiation in our VE- and PTX-treated cohort. Following analysis of patient data, the lowest incidence of pneumonitis was experienced at 3 months post-treatment, with 11% of our cohort experiencing grade 1 pneumonitis and 7% experiencing grade 2 pneumonitis. No participants reported post-treatment toxicities associated with either PTX or VE during post-treatment follow-up visits. In accordance with proportional odds regression ( Table 4 ), toxicity was significantly higher at 6 months than at 3 months (proportional odds ratio = 2.64, P = .029), but no statistically significant associations between toxicity and other demographic/clinical factors were found. The model used for Table 4 uses the ordinal grade values (0 vs 1 vs 2 vs 3), not a binary cut-off. The proportional odds effect reflects the odds of an increase in grade.
Toxicity Results at 3, 6, and 12 Mo Post-Treatment
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 3 < 30% | Missing | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| 3 mo | 22 | 79 | 3 | 11 | 2 | 7 | 0 | 0 | P = .001 | 13 |
| 6 mo | 12 | 55 | 5 | 23 | 4 | 18 | 1 | 5 | P = .004 | 18 |
| 12 mo | 7 | 54 | 6 | 46 | 0 | 0 | 0 | 0 | P = .010 | 27 |
Trends in grades of pneumonitis at 3, 6, and 12 mo post-treatment.
Results From Proportional Odds Model on Toxicity Grade
| Odds Ratio | Confidence Interval | P Value | Missing Observations | |
|---|---|---|---|---|
| Time (mo) | .005 | |||
| 3 | Ref | |||
| 6 | 2.64 | 1.10–6.31 | .029 | |
| 12 | 2.63 | 0.97–7.14 | .057 | |
| ECOG | ||||
| 0 | Ref | |||
| 1 or higher | 0.87 | 0.38-1.97 | .733 | |
| Previous radiation dose (Gy) | 0.84 (10-unit change) | 0.53-1.35 | .477 | 10 |
| Interval between doses >12 mo | 0.56 | 0.22-1.53 | .226 | 8 |
| Central tumor (vs peripheral) | 1.97 | 0.62-6.25 | .249 | 4 |
| Noncompliant | 2.22 | 0.57-8.65 | .251 | |
| Pretreatment FEV1 | 2.00 (1-unit change) | 0.38-10.40 | .410 | 27 |
Model is fit using all available toxicity grades (n = 62), and random effects are used to control for correlation between multiple scores from the same individual. The effect for each variable is assessed by controlling for time (3 mo, 6 mo, 12 mo).
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FEV, forced expiratory lung volume.
Tumor Recurrence and Overall Survival
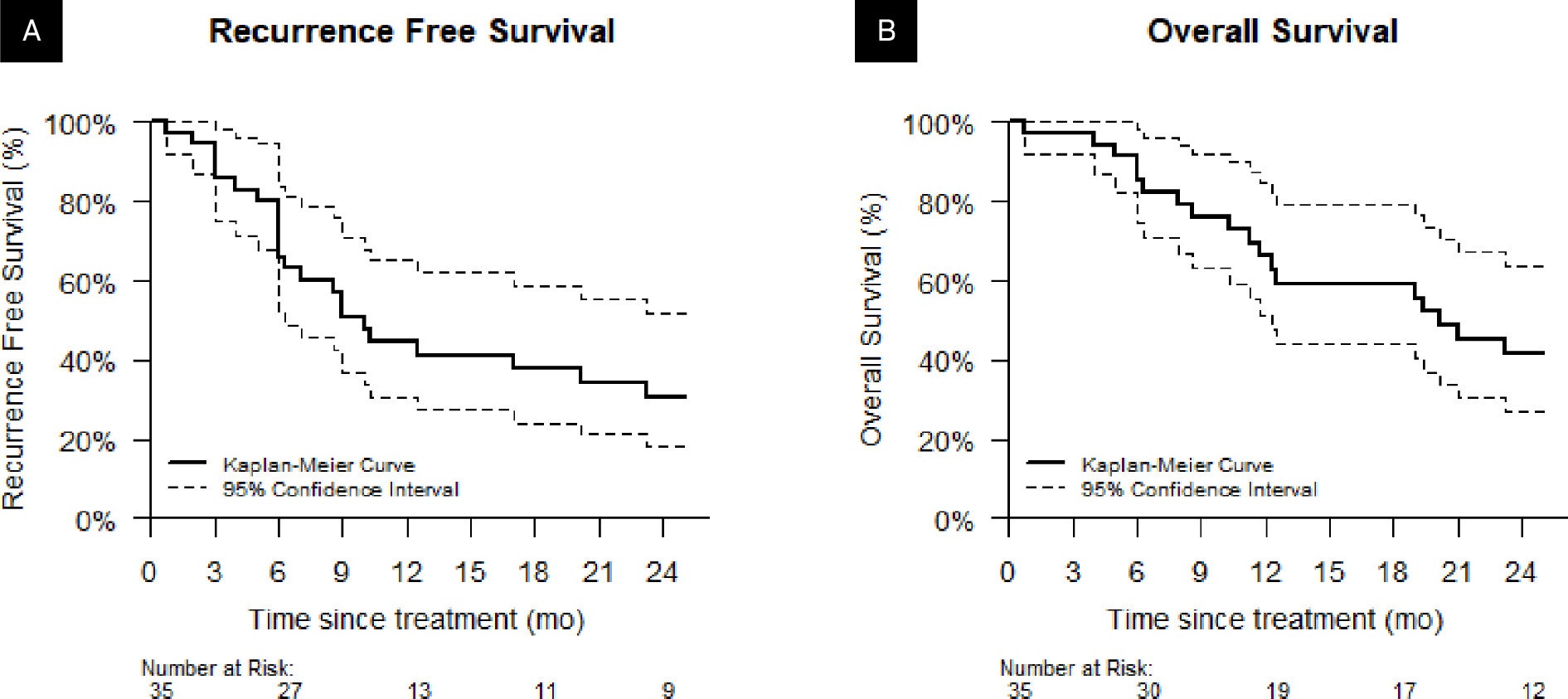
Of our initial cohort of 40 patients, 5 were lost to follow-up after treatment completion and were excluded from the survival analysis. Of the remaining 35 patients, 14 (40%) had an observed recurrence, 2 of which were local, 6 of which were regional recurrences, and 6 of which were distant. 27 (77%) patients died during the study. Figure 2 reports the Kaplan-Meier plots for RFS and OS. Median RFS was 10 months (95% CI 6-30 mo) and median OS was 20 months (95% CI 12-50 mo). The 1-year RFS rate was 44% (31%-55%) and 1-year OS was 66% (51%-85%).
Kaplan-Meier estimates for (A) recurrence-free survival and (B) overall survival.
Discussion
SABR is a valuable reirradiation modality for patients with new or recurrent lung malignancies. VE and PTX are well-tolerated medications that are potentially efficacious in preventing grade 3 and higher rates of radiation-induced pneumonitis. 92% of our patients were medication-compliant and reported little or no side effects associated with VE and PTX. Of our cohort of 40 patients, only one experienced grade 3 pneumonitis, with most experiencing grade 0 pneumonitis. Median OS was 20 months, and median RFS was 10 months. To date, our trial is the first to assess the utility of these agents for patients being reirradiated via SABR for locoregionally recurrent disease or new primary malignancies.
Data regarding the rates and predictors of radiation-induced pneumonitis in the setting of primary radiation vs reirradiation have been published previously. In the primary setting, Schallenkamp et al16 reported a rate of 13% in grade 2 or greater pneumonitis in the setting of conventional radiation, which may be related to the percentage of total lung volume minus gross tumor volume receiving ≥10 Gy. Accordingly, lower radiation doses to larger volumes of lung may induce a significant, symptomatic, and more aggressive inflammatory response than that experienced with higher doses of radiation. Patients with subclinical interstitial lung disease (ILD) have been found to be at greater risk for higher grades of pneumonitis—of 87 patients with subclinical ILD, 11.5% experienced grade 3 pneumonitis, 3.4% experienced grade 4 pneumonitis, and 5.7% experienced grade 5 pneumonitis, and the cumulative incidence of these high grades of pneumonitis was significantly higher in patients with subclinical ILD involving ≥25% of the lungs (46.1% vs 16.3%, P = .004).17
Systemic therapies may similarly influence the rates of grade 3 or higher primary radiation-induced pneumonitis. Neoadjuvant therapy with gemcitabine has been associated with a significantly higher incidence of grade 3 or higher pneumonitis (32.3% vs 13.3%, P = .023).17 Of 106 patients treated with concurrent chemoradiation, pneumonitis ≥grade 2 occurred in 47 patients (44.3%), and pneumonitis ≥grade 3 occurred in 6 (5.7%).18 Among this cohort, adjuvant therapy with immune checkpoint inhibitors (durvalumab, atezolizumab, pembrolizumab, or nivolumab) significantly influenced the rate of grade 2 or higher pneumonitis but not rates of grade 3 or higher pneumonitis.18
Understandably, toxicities are more prominent in the setting of reirradiation with SBRT. Data from the MD Anderson Cancer Center suggest a rate of grade 3 pneumonitis ranging from 18.9%19 to 30%.4 Rates of toxicity may be influenced by the following factors: ECOG score of 2 or 3, an FEV1 ≤ 65%, a previous PTV spanning the bilateral mediastinum, and V20 ≥ 30% on composite (previous RT + SABR) plans.19 Others report comparable rates of toxicity, with 26% of a cohort experiencing grade 3 or greater toxicity. However, among this same cohort, 14% ultimately died from a post-treatment lung-related event other than bacteria-associated pneumonitis or disease progression. Subsequent chemotherapy, rather than radiation therapy factors such as total dose, lung dose, or interval between doses, was significantly related to such lethal events ( P = .009).20 Finally, out of a cohort of 70 patients followed throughout their primary and reirradiation treatments, 15 (21.4%) developed pneumonitis ≥grade 3 in the reirradiation setting.21
Given the prominence of symptomatic pneumonitis, particularly in the setting of reirradiation, clinicians should aim to limit such toxicities while maintaining ideal dose prescription and treatment parameters. VE and PTX are tools that can potentially help treating physicians achieve this goal. PTX is a nonselective phosphodiesterase inhibitor that increases intracellular cyclic GMP levels, resulting in greater flexibility of the red blood cell and leukocyte membrane. This permits greater passage of oxygen and nutrients through microvessels and, ultimately, into tissue. VE is a fat-soluble vitamin principally found in the cell membrane. It is also thought to contribute to membrane flexibility and may offer vascular protection and anti-inflammatory and antifibrosis capabilities.22 Notably, they are well tolerated; the most common side effects of each medication are nausea, vomiting, and diarrhea, none of which were experienced by patients in our cohort.9, 23 Several studies have evaluated the efficacy of these agents, albeit outside of the setting of reirradiation with SABR for thoracic malignancy. For patients with breast cancer, randomized controlled trials suggest that VE and PTX lessen the degree of radiation-induced fibrosis but may not significantly impact OS or progression-free survival.10, 24 They are similarly beneficial in the setting of primary radiation for lung cancer, with PTX and VE patients experiencing a lower burden of lung toxicities compared with placebo-treated patients.11
Of note, a brief discussion is warranted regarding the concerns that have arisen from recent studies over the detrimental effects of VE supplementation. A meta-analysis of 19 trials found increased overall mortality in patients taking high doses of VE (over 400 IU daily for at least 1 y).25 Another analysis indicated increased mortality from VE and β-carotene supplementation in the prevention of gastrointestinal cancers.26 Additionally, the Selenium and Vitamin E Cancer Prevention Trial demonstrated that 400 IU VE supplementation raised prostate cancer rates in men after a follow-up of at least 7 years.27 However, these findings are based on long-term and/or high-dose VE use, contrasting with our trial’s short-term, standard supplementation. Moreover, our cohort differs significantly from those studied, possibly explaining the benefits associated with supplementation for patients undergoing reirradiation who are under significant physiological stress, which in turn may help explain the pneumo-protective impact of short-term antioxidant use. Lastly, variations in supplement quality, alternative isoforms of VE, patient-to-patient CYP450 metabolism, and drug-drug interactions impacting VE metabolism—none of which have been accounted for in these studies—may also influence individual VE susceptibilities and outcomes, further complicating interpretations.
Future studies are warranted to further address the tolerability and effectiveness of VE and PTX. Double-blind, randomized controlled trials will help better elucidate the clinical utility of these pharmaceutical agents. Additionally, studying these agents in the setting of other radiotherapeutic treatments for other malignancies such as gastrointestinal cancers, head and neck cancers, and/or gynecologic and urologic cancers could be beneficial.
Strengths and Limitations
Given the simple design of this prospective study, we were able to clearly identify and accurately evaluate our principal outcome of rates of grade 3 pneumonitis in our cohort of patients with recurrent NSCLC. VE and PTX are well tolerated, allowing for a high compliance rate and suggesting they can be safely and similarly studied in the setting of reirradiation for other disease sites. Regarding limitations, as this was a single-arm clinical trial, we are unable to compare the utility and efficacy of VE and PTX compared with placebo, which would be afforded in the design of a randomized, double-blind, placebo-controlled trial. Our sample size is small and from a single institution; recruiting a more diverse cohort from across the United States would be beneficial. Also, some participants were lost to follow-up at different times post-treatment, possibly introducing a bias in our results if those participants ultimately developed different health outcomes secondary to late toxicities associated with the study drugs and/or radiation course compared with the participants who remained in the study.
Finally, further collecting pretreatment physiological and radiotherapeutic data to help better identify predictors of toxicity, a topic that was briefly explored in our discussion, would be warranted. Particularly, obtaining accurate pretreatment isodose volumes in addition to pretreatment dose/fractionation would be warranted. Many of the patients in this study were referrals from rural communities, where they may have received incomplete treatment for their primary malignancies, thus explaining the wide range of previously administered radiation dose. We did not have access to data for the specific circumstances dictating original radiotherapeutic dose selection or other pretreatment factors that may have influenced each patient’s response and/or compliance to the study at hand. We also do not have pretreatment radiotherapeutic data beyond that of prior dose and fractionation to correlate with reirradiation toxicity.
Conclusion
PTX and VE are safe interventions that may prophylactically reduce rates of grade 3 pneumonitis for patients receiving subsequent SABR for recurrent NSCLC. Additional studies should be performed to evaluate the use of PTX and VE in the retreatment setting.
References
Citation
Willett A, Silverman C, Woo S, Gaskins JT, Dunlap N. The Effect of Pentoxifylline and Vitamin E in Preventing Grade 3 Radiation Pneumonitis: A Single-Arm, Phase II Prospective Study. Appl Radiat Oncol. 2025;(2):1 - 9.
doi:10.37549/ARO-D-25-00012
June 1, 2025