First Patient Dosed in Renal Cancer Theranostics Phase II Study
 The first patient has been dosed in the ‘STARLITE 2' Phase II study of Telix Pharmaceutical’s investigational renal cancer therapy, TLX250, at Memorial Sloan Kettering Cancer Center in New York.
The first patient has been dosed in the ‘STARLITE 2' Phase II study of Telix Pharmaceutical’s investigational renal cancer therapy, TLX250, at Memorial Sloan Kettering Cancer Center in New York.
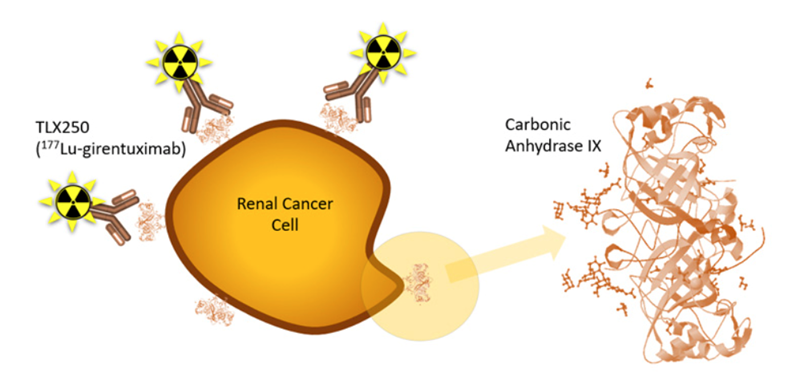
STARLITE 2 (NCT05239533) will assess the efficacy of TLX250 targeted radiation in combination with immunotherapy for clear cell renal cell carcinoma (ccRCC), the most common and aggressive form of kidney cancer. TLX250 targets carbonic anhydrase IX (CA9), a protein that is highly expressed in patients that are likely to demonstrate a more limited response to cancer immunotherapy. The concept is that low doses of targeted radiation can potentially overcome immune resistance – or "immune prime" a tumor and therefore make it more responsive to cancer immunotherapy.
This Phase II study, in patients who have progressed following prior immunotherapy, will evaluate TLX250-delivered radiation in combination with the anti-PD-1 immunotherapy Opdivo (nivolumab). The primary endpoint is to determine the safety and efficacy of combination therapy with TLX250 as assessed by the tumors responding to the Telix therapy versus the current standard of care alone. Telix's investigational companion imaging agent TLX250-CDx (Zr-DFO-girentuximab) will also be used in the study to image CA9 expression. The single-arm investigator-led study is expected to enroll approximately 30 patients.
Telix Chief Medical Officer, Dr Colin Hayward noted, "The integration of precision nuclear medicine and medical oncology is underway and Telix is at the forefront of this movement to develop personalized products and patient-friendly regimens. We wish to express our gratitude to Dr Darren Feldman and his clinical team, as well as the patients who will contribute to this ground-breaking study."