Extended PSMA-Targeted Therapy is Safe and Effective in Prostate Cancer Patients
 A new study published in The Journal of Nuclear Medicine reports that extended prostate-specific membrane antigen (PSMA)-targeted radiopharmaceutical therapy (177Lu-PSMA) beyond six cycles is an effective and well-tolerated treatment for metastatic castration-resistant prostate cancer patients. Selected patients who received extended treatment—either continuously or following a treatment break—experienced a favorable median survival of 31.3 months from the first administration.
A new study published in The Journal of Nuclear Medicine reports that extended prostate-specific membrane antigen (PSMA)-targeted radiopharmaceutical therapy (177Lu-PSMA) beyond six cycles is an effective and well-tolerated treatment for metastatic castration-resistant prostate cancer patients. Selected patients who received extended treatment—either continuously or following a treatment break—experienced a favorable median survival of 31.3 months from the first administration.
“Patient response to PSMA-targeted radiopharmaceutical therapy is highly variable in depth and duration of response,” said Wolfgang P Fendler, MD, vice chair of the Department of Nuclear Medicine at the University Hospital Essen, in Essen, Germany. “It may be beneficial to extend the use of 177Lu-PSMA, however, systematic data on safety and the antitumor effect of 177Lu-PSMA radiopharmaceutical therapy beyond six cycles is scarce.”
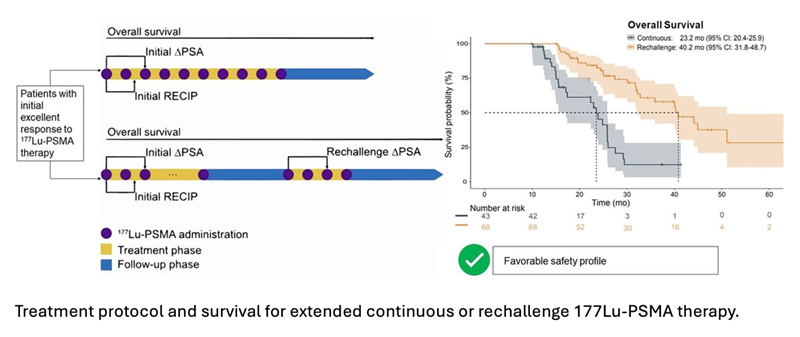
The multicenter retrospective analysis included 111 metastatic castration-resistant prostate cancer patients who received more than six cycles of 177Lu-PSMA. Forty-three patients (38.7 percent) received continuous 177Lu-PSMA treatment and 68 (71.3 percent) received rechallenge treatment after a therapy break of at least four months. Researchers assessed overall survival, prostate-specific antigen (PSA) decline, PSMA PET response, and adverse events to determine the safety and efficacy of extended 177Lu-PSMA therapy.
Overall survival from the initiation of 177Lu-PSMA therapy was 23.2 months for the continuous treatment group and 40.2 months for the rechallenge group; the median overall survival for both groups was 31.3 months. The PSA decline of more than 50 percent and the initial partial response rate on PSMA PET was significantly higher in the rechallenge group than in the continuous treatment group. Rates of grade three to four toxicity were comparable between both groups.
“This study represents the inaugural multicenter investigation of extended 177Lu-PSMA therapy, encompassing up to 13 cycles of treatment, continuously or following a treatment break. Dose reduction and discontinuation rates due to adverse events were low and patients achieved durable responses after treatment pause,” said Tugce Telli, MD, researcher at University Hospital Essen. “Given the limited treatment options for metastatic castration-resistant prostate cancer, the extension or retreatment of 177Lu-PSMA therapy beyond six cycles may be a valuable option for patients who initially have a good or excellent response to treatment.”