Eradicating Cancer Stem Cells Using High Linear Energy Transfer Radiation Therapy Part 2: LET Painting and Other Advanced Techniques
Affiliations
- 1 Department of Radiation Oncology, Mayo Clinic Florida/Mayo Clinic Comprehensive Cancer Center, Jacksonville, FL
- 2 Department of Radiation Oncology, Mayo Clinic Rochester/Mayo Clinic Comprehensive Cancer Center, Rochester, MN
- 3 Department of Radiation Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY
- 4 Department of Radiation Oncology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 5 Charité – Universitätsmedizin Berlin, Berlin, Germany
- 6 German Cancer Consortium (DKTK) and German Cancer Research Center (DKFZ), Heidelberg, Germany
- 7 Department of Radiology and Precision Health Program, Michigan State University, Lansing, MI
- 8 Department of Radiation Oncology, Ohio State University Comprehensive Cancer Center, Columbus, OH
- 9 Department of Radiation Medicine, Northwell Cancer Institute, New Hyde Park, NY
- 10 Biophysics Department, GSI Helmholtzzentrum für Schwerionenforschung, Darmstadt, Germany
CME credits are available here.
Linear energy transfer (LET), a measurement of ionization density, tracks the radiobiological potency of any given course of therapeutic radiation and its efficacy in killing cancer cells. As opposed to the low LET of photon and proton therapy, high LET charged particle therapy can overcome multiple mechanisms of resistance to effectively treat radioresistant tumors. A robust basic science literature demonstrates enhanced direct cancer stem cell (CSC) sterilization with increasing LET along with indirect mechanisms of tumor control such as immunogenesis. Such a strategy has yet to be implemented in clinical practice in the absence of an effective means of targeting CSCs without risking unacceptable harms to patients. In Part 2 of this 2-part series, we review newly emergent functional imaging technologies in conjunction with existing techniques of spatial fractionation and capabilities for multi-ionic therapy that hold promise as a means of translating the biological potential of high LET therapy into clinical protocols for effective anti-CSC therapy.
Keywords: LET, linear energy transfer, particle therapy, carbon ion therapy, heavy particle therapy, radiobiology, radiation physics, spatial fractionation, ion therapy
Introduction
Building upon the physical and radiobiological advantages of high linear energy transfer (LET) discussed in “ Eradicating Cancer Stem Cells Using High Linear Energy Transfer Radiation Therapy Part 1: Physics and Radiobiology ,” this article examines the clinical translation of heavy particle therapy (HPT), especially via LET painting, to overcome cancer stem cell (CSC)-related resistance. It explores how multi-ion strategies, spatial fractionation, and biologically guided planning enable targeted dose intensification to resistant subregions while sparing normal tissues. In doing so, it addresses not only technical implementation but also economic, logistical, and immunological challenges and opportunities.
Clinical Implementation of HPT
LET Painting for Treatment Planning Adaptive to Tumor Heterogeneity
The peak-to-plateau ratios of HPT, based on changes in LET over the course of the beam path, enable “LET painting” or “kill painting”--that is, the conformal and selective escalation of LET to a defined subvolume within a larger target volume that is otherwise treated with lower LET.1 - 6 LET painting was developed initially to overcome the radioresistance of hypoxic tumor subregions using functional tracers such as18 F-FMISO for guidance.3 More recently, ionic copper-based hypoxia tracers such as64 Cu-ATSM and64 Cu-NOTA have been well-validated in cell and animal experiments. Though still early in their clinical development, these agents may allow for a more direct means of CSC targeting, not merely via hypoxia surrogacy, but through biological affinity for stemness markers such as CD133.7 - 10 This shift suggests the potential for LET painting to transition from targeting microenvironmental resistance (i.e., hypoxia) to directly mapping and ablating CSC populations.
Conjugation of64 CuNOTA to antibodies targeting the AC133 epitope of CD133 has enabled high-contrast detection of CD133-expressing gliomas in murine xenografts using both PET and near-infrared fluorescence molecular tomography.11 Direct detection of CSCs using superparamagnetic iron oxide nanoparticles conjugated to appropriate homing moieties to CSC biomarkers such as AC133, AC141, CD44v6, and CD109 is under preclinical investigation by the present authors. This approach may allow for CSC delineation at the spatial resolution conditions of MRI.12
If successfully translated to the clinic, such functional imaging could support the identification of both high-biological risk tumor volumes (HRTVs) for targeted LET escalation, while defining low-biological risk tumor volumes (LRTV = GTV – HRTVs) for standard or de-escalated treatment. The most precise and conformal LET painting strategies may ultimately rely on treatment plans incorporating combinations of different ionic beams.10, 13 The addition of multiple ion species to a plan augments LET ranges for given doses, volume sizes and shapes, and desired gradients.
The potential for de-escalated therapy to LRTVs is no less important than escalated therapy to HRTVs in establishing HPT as a clinically viable strategy for improving outcomes in resistant cancers. Just as HPT is uniquely capable of sterilizing the most resistant malignant cells, its radiobiological potency creates peril for even those normal tissues capable of resisting injury by high doses of conventional radiation therapy (RT). Moreover, normal tissue injury as a result of HPT is more likely to be irreparable than that caused by conventional therapy.
A cautionary precedent can be found in a clinical trial in neon ion radiation therapy (NIRT) for glioblastoma (GBM) conducted at the Lawrence Berkeley Laboratory (LBL). Although the trial was terminated prematurely due to the facility’s closure, early outcomes included tumor control and survival nominally comparable or superior to that seen in the modern treatment of GBM. However, these were accompanied by high grade late toxicities including potential treatment-related grade 5 toxicity in patients whose tumors had been controlled.14 LET painting is thus an essential treatment planning strategy for safe clinical implementation of HPT with significantly augmented therapeutic ratio vs that of conventional RT.
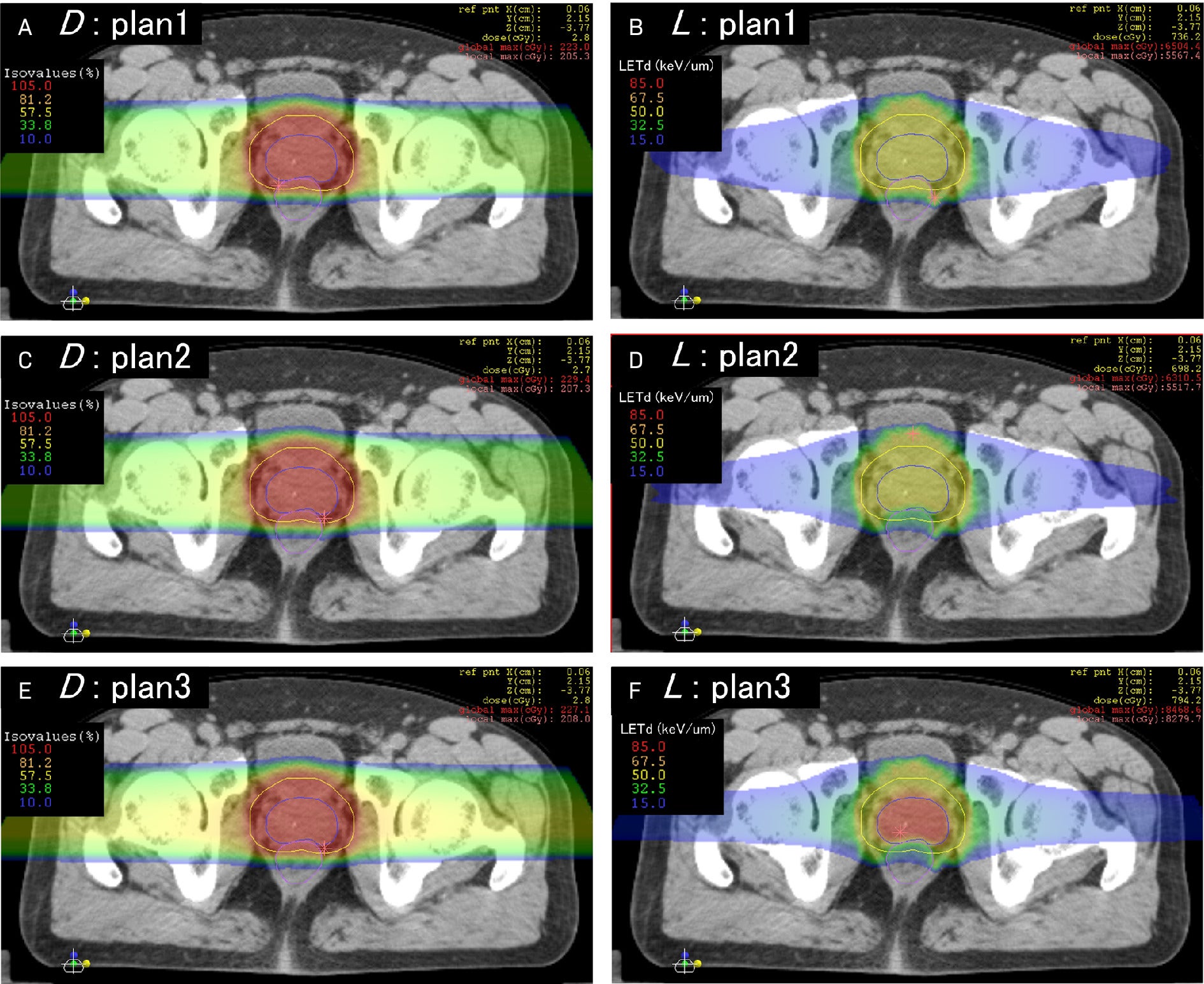
Apart from protons and carbon ions already established in clinical use, research has been done on other species, including helium, lithium, oxygen, and neon ions.10, 13 - 19 Clinical helium ion therapy has commenced at the Heidelberg Ion Therapy Center (HIT, Germany), with clinical oxygen ion therapy under development.20 Mayo Clinic Florida likewise plans to attain capacity for combination heavy ion therapy. Unlike other oncologic therapies, HPT offers modularity in dose distribution and potency, facilitating the individualization of therapeutic prescription, including at the level of intra-tumor heterogeneity.21 - 24 Even before the clinical availability of multi-ionic radiation therapy (MIRT), the intensity-modulated composite particle therapy (IMPACT) and spot-scanning hadron arc (SHArc) models at the National Institute of Radiological Sciences (NIRS, Japan) and HIT, respectively, have been validated in Monte Carlo simulation for multi-ion treatment planning using LET painting and direct LET-based optimization extended to cover treatment with any combination of protons, helium ions, carbon ions, oxygen ions, and neon ions ( Figure 1 ).4 - 6, 25 - 31 The greater the variety of LET spectra from different ions for treatment, the steeper the achievable LET gradients, the less the LET delivered to one voxel forces the LET range deliverable to adjacent voxels. Improved conformality of LET distributions to irregularly shaped targets such as those delineated by functional imaging enables painting of LET gradients onto planning imaging at will.3 - 5, 10, 13, 31 Preclinical and clinical studies of MIRT are summarized in Table 1 .
Representative linear energy transfer (LET) painting case reprinted with permission from Inaniwa et al.6 The left column represents RBE-weighted dose (D), and the right column represents dose-averaged LET (L) for 3 prostate cancer plans generated via the intensity-modulated composite particle therapy technique, with panels A-B, C-D, and E-F representing the dose and LET variables for the same plans, respectively. All plans are isodosimetric with variations in LET distribution. Note in plan 1 the uniformly higher LET in the anterior rectum with an aberrant LET hot spot along the left anterolateral rectal wall, outside the target, that would go undetected on pure dosimetric analysis. The LET distribution to the organs at risk is corrected in plans 2 and 3, and LET to the target is successfully escalated isodosimetrically in plan 3.
Studies of Multi-Ionic Radiation Therapy
| Study | Institution | Ion Species | Variable(s) Under Study |
|---|---|---|---|
| Inaniwa et al27 | NIRS (Chiba, Japan) | Helium, carbon, oxygen, neon | Validation of SMK relative biological effectiveness (RBE) model on undifferentiated carcinoma and PDAC cells |
| Sokol et al10 | GSI (Darmstadt, Germany) | Helium, oxygen | Multi-ionic LET painting for overcoming hypoxia radioresistance effects |
| Inaniwa et al6 | NIRS (Chiba, Japan) | Proton, helium, carbon, oxygen | LET optimization via intensity-modulated multi-ionic radiation therapy (IMPACT) |
| Mein et al25 | HIT (Heidelberg, Germany) | Helium, carbon, oxygen, neon | LET optimization via multi-ionic hadron arc therapy (SHArc) |
| Inaniwa et al26 | National Institutes for Quantum Science and Technology (QST) (Chiba, Japan) | Helium, carbon, neon | Adaptation of multi-ionic therapy planning via SMK to account for oxygen-dependent cell responses (OSMK) |
| Sakata et al28 | QST (Chiba, Japan) | Combination helium + oxygen vs carbon + neon | Silicon microdosimetric validation validation of MIRT planning |
| Inaniwa et al29 | QST (Chiba, Japan) | Helium, carbon, oxygen, neon, silicon | Refinement of OSMK accounting for greater range/variation in LET over additional cell lines |
| Inaniwa et al30 | NIRS (Chiba, Japan) | Helium, carbon, oxygen, neon | Correction of dose calculations for MIRT |
| Kopp et al32 | HIT (Heidelberg, Germany) | Proton, helium, carbon | Initiation development of treatment planning system for MIRT |
| Inaniwa et al26 | NIRS (Chiba, Japan) | Helium, carbon, oxygen, neon | Validation of MIRT optimization via lung substitute material |
| Masuda et al, 202533 | QST (Chiba, Japan) | Carbon, oxygen, neon | Retrospective validation of LET optimization to GTV for MIRT in patients with head and neck cancer (n = 16) |
Abbreviations: GSI, GSI Helmholtzzentrum für Schwerionenforschung; GTV, gross tumor volume; HIT, Heidelberg Ion Therapy Center; IMPACT, intensity-modulated composite particle therapy; LET, linear energy transfer; MIRT, multi-ionic radiation therapy; NIRS, National Institute of Radiological Sciences; OSMK, oxygen-effect-incorporated stochastic microdosimetric kinetic model; PDAC, pancreatic ductal adenocarcinoma; RBE, relative biological effectiveness; SHArc, spot-scanning hadron arc model; SMK, stochastic microdosimetric kinetic model.
HPT Planning: Spatial Fractionation to Maximize CSC Kill and Minimize Toxicity
LET painting with MIRT has particularly favorable properties for targeting CSCs in pancreatic ductal adenocarcinoma (PDAC) and GBM. In GBM, radiation volumes often extend to near-hemispheric dimensions due to the tumor’s spread, while in PDAC, the proximity of critical organs at risk (OARs), particularly the duodenum, necessitates strict dose limitations or reductions.34 - 36
Although mono-ionic beams at ultra-high LET can sterilize CSCs throughout tumor organoids or xenografts without spatial discrimination, achieving uniform coverage to a CSC-ablative LET level across the gross tumor volume (GTV) in patients is substantially more challenging. The CLEOPATRA phase II randomized clinical trial, aiming to improve overall survival in GBM using carbon ion radiation therapy (CIRT), underscores this limitation. Of the total prescribed RT dose, only 18 Gy (RBE)—roughly 25%—was delivered with CIRT, with the remaining dose administered using conventional low LET photons, most likely due to increasing rates of toxicities such as high-grade radiation necrosis increasing with irradiated volume and dose, even at low LET.37, 38
Although the final results from CLEOPATRA have not yet been published, it is plausible that the CIRT component was insufficient to yield a measurable survival benefit. Considering the standard curative-intent dose of 60 Gy, it is probable that, even if a signal exists, too little CIRT was given to capture it. In other words, directing HPT to entire GTVs frequently necessitates compromising dose and/or LET below the necessary threshold for optimal biological effect on the tumor and CSCs.
In our view, the superior strategy is the one that LET painting enables, namely partial volume ablation at high dose/high LET combinations, with the remainder of relatively radiosensitive and non-clonogenic volumes treated with a lower intensity/less toxic intervention, or even none at all. The concept of therapeutic modulation across tumor subregions—central to LET painting—already has a precedent in clinical practice through spatial fractionation, which is employed in the palliative setting for refractory, unresectable disease.39 Though not technically a novel approach, spatially fractionated radiation therapy has resurged in the literature with improved delivery mechanisms and the utilization of immunotherapy in cancer care. Multiple techniques appear today; most prevalent is grid or lattice radiation therapy, painting a milieu of dose upon a tumor with focal peaks in dose surrounded by low-dose valleys, demonstrating marked response.40 This approach potentially generates an immunological reaction to targeted tumor tissue, but the impact on CSCs is unknown. The grid is characterized by dose spheres with relatively random distribution within a larger, bulkier tumor, allowing the tumor tissue to provide a safe margin between dose peaks and OARs.
Focusing on immunological development and enhanced dose deposition in hypoxic CSC regions, the PArtial Tumor Irradiation Targeting HYpoxic Segment (PATHY) approach aims to selectively irradiate tumors in a more directed manner. PATHY is directed to one or more “Bystander Tumor Volume” (BTVs) defined as marginal reductions within the GTV, with the aim of sparing the surrounding peritumoral immune microenvironment (PIM) by subjecting it to tight formal constraints as a contoured OAR.41, 42 PATHY using photon stereotactic body radiation therapy (SBRT) and proton beam therapy (PBT) has demonstrated success at dramatic volumetric reductions of the GTV through bystander effects.42 - 44 Refined by Tubin and colleagues at the MedAustron Ion Therapy Centre (Wiener Neustadt, Austria), PATHY is currently administered exclusively as a technique of 3-fraction daily CIRT.
A transition is now underway from simple Boolean-based geometric reductions of the GTV to biologically informed target delineation, with the BTV increasingly defined through functional hypoxia tracer imaging, such as with ⁶⁴Cu-ATSM PET.45 Carbon-PATHY achieves significant treated tumor response and demonstrates methodologically validated macroscopic abscopal responses of unirradiated nodal and distant metastases.42, 46 At the preclinical stage, a recent experiment of murine xenografts of breast cancer showed that microtargeted partial CIRT fields determined by hypoxia PET imaging demonstrated an equivalent abscopal response to that of whole-volume carbon irradiation on non-irradiated grafts.47
Collectively, these murine and human data support the potential immunogenicity of confining HPT target volumes to select HRTVs. Similar minibeam techniques have been developed for use with heavier ions, particularly neon. Spatially fractionated NIRT has succeeded in vivo in murine models at inducing brisk reoxygenation and tumor-killing within hypoxic regions while sparing severe skin toxicity compared with broad-beam NIRT.48 - 51 These promising early findings raise the prospects of immediate, definitive clinical applications for revived very high LET therapy, approaches that avoid the prohibitive late toxicities observed in the initial NIRT experiments at LBL, which curtailed its clinical adoption.14, 17 - 19 Such therapy would involve LET values exceeding conventional relative biological effectiveness (RBE)-based optimization thresholds, but in doing so may approach, and help define, the LET levels required for optimized anti-CSC therapy.
HPT and Immunotherapy: Turning Cold Tumors Hot
HPT may play a vital role in achieving more favorable outcomes for immunotherapy in cancers such as PDAC and GBM for which efforts in immunotherapy to this point have yielded frustratingly little benefit. PDAC is an archetypal immunologically “cold” tumor, demonstrating poor response to immune checkpoint inhibitors (ICIs) despite overexpression of PD-L1 and CD47. This resistance stems from complex genetic and epigenetic feedback mechanisms that collectively promote an immunosuppressive tumor microenvironment (TME); enhance clearance of cytotoxic agents and repair of cytotoxic damage; block activation, expansion, and infiltration of cytotoxic and pro-immune lymphocytes; suppress antigen recognition; and enable evasion and suppression of peripheral phagocytes.52, 53 Likewise, GBM appears to be immunologically cold with expression of PD-L1 found in a majority of tumor specimens and correlated with M2-polarized peripheral tumor-associated macrophages (TAMs), reduced lymphocyte infiltration, chemoresistance, and overall poor prognosis, ultimately failing in clinical evaluation to yield the hypothesized therapeutic gains for ICI based on crude PD-L1 expression on pathology.54 - 57
Emerging data suggest that the failure of immunotherapy in PDAC and GBM may reflect, at least in part, the central role of CSCs. In GBM, PD-L1 overexpression is driven by β-catenin—the terminal transcription factor of the canonical Wnt pathway and a key mediator of CSC–epithelial-to-mesenchymal transition (EMT) crosstalk in both PDAC and GBM. β-Catenin functions ubiquitously as an effector of stemness and EMT programs. Experimental data in GBM demonstrate strong co-expression of CD133 and β-catenin, with CD133 knockout resulting in the suppression of β-catenin, supporting a causal link downstream of CD133.58 - 62
Moreover, GBM cell culture analysis has found an inverse correlation between CD133 expression and CD4/CD8 infiltration while surgical specimen analyses have found M2 polarization and immunosuppressive microglia induction in GBM to be a product of the same CD133-activated Akt-Wnt interchange with redundant additional promotion by the CSC-associated TGF-β pathway.63 - 65 Similarly, analysis of PDAC resection specimens has demonstrated a correlation between high PD-L1 expression and expression of both CD133 and CD44 that is lost when CD8 lymphocyte infiltration is high.66 PDAC data further demonstrate an immunosuppressive M2 polarization of TAMs, low TME levels of CD4 and DC infiltration, relatively high T H 17 levels, high T H 2:T H 1 ratio, and an anti-immune cytokine balance.67 - 70
Collectively, these findings point to a deep relationship between the phenomenon of immunological coldness and the PDAC- and GBM-CSC biology mediated by CD133 and CD44. Likewise, the circulating tumor cells (CTCs) responsible for metastasis are the result of EMT-mediated transformation and extravasation; the mobile immune-privileged circulatory routes, docking and colonization of distant organs necessary to complete the metastatic arc are only possible for CTCs acquiring a sufficient range and degree of stemness properties.71 - 78 Preclinical evidence of the unique efficacy of high LET therapy in curbing CSC biology suggests the very same mechanisms may overcome native tumor resistance to immunotherapy, while the clinical demonstration of abscopality in the PATHY experience points to spatially fractionated HPT as the technical means by which local RT can be harnessed to achieve systemic tumor control effects.42 In this context, maximizing the immunological benefits of HPT may require more than ICI alone. Recently developed CAR NK cell therapies not only yield more efficient direct tumor cell killing than CAR T cells, particularly in solid tumors, but also mimic the action of HPT in turning immunologically cold TMEs hot via activation of cytotoxic T cells and induction of M1 polarization, all without the inflammatory toxicities associated with CAR T therapies and which might be feared to result from combining HPT and ICIs.79 - 84 Finally, the immunogenic mechanism of HPT, that is, cytosolic exposure of high volumes of tumor DNA, lends itself well to ongoing work in the development of personalized cancer vaccines, showing promising results in GBM and PDAC in early-stage clinical trials.85, 86
Biological Optimization of HPT Physics: Calibrating the Instruments of CSC Eradication
Currently, the LET thresholds required for complete CSC sterilization remain unknown. Available data consistently demonstrate that CIRT enhances CSC killing, suppresses EMT, and reduces the oxygen enhancement ratio (OER), and they suggest a general relationship between LET, OER, and clonogenic survival. However, none of these studies were designed to achieve, nor did they achieve, complete CSC eradication. Instead, they relied on traditional RBE determination based on D values (the dose required to achieve 90% cell kill), which is useful for comparing CSC sensitivity between CIRT and photon or proton radiation but insufficient for defining the LET and dose conditions necessary to achieve CSC extinction. Moreover, these studies did not develop a framework to characterize clonogenic extinction in terms of changes in CSC biomarker expression as a function of LET; they simply reported expression changes at a limited number of dose–LET combinations.87 - 106 Notably, they were also silent on non-local, indirect mechanisms of CSC or tumor control, such as immunogenic self-potentiation.
Most importantly, the LET range across these studies is wider than the delta between proton/photon LET and the lowest LET values of the carbon ion Spread Out Bragg Peak (SOBP) by multiples still inadequate to the task even at its upper boundary; the aggregate modal LET is 50 keV/µm but its selection is never justified for the treatment of CSCs within published material—50 keV/µm is a typical central tendency of whole tumor coverage by a carbon ion SOBP—and it is associated only with enhanced CSC killing, taking clear surviving fractions to be a given a priori.88, 90, 93 - 96, 102, 104, 106 One significant report, drawing upon a model determining an LET U value of maximum kill efficiency, insists that LET should not exceed 100 keV/µm because of overkill effects and diminishing RBE.101, 107 Conversely, in preclinical settings, outright CSC eradication has been achieved by carbon ion irradiation at an arbitrarily determined 120 keV/µm delivered over clinically irrelevant dose/fraction regimens, illustrating the vast gap between the prominence of overkill in the literature and its actual clinical impact.98, 99
Underkill of CSCs, unlike overkill, is an active clinical issue with implications for the prognosis of PDAC or GBM at any stage of disease and the expected 100% rate of failure for every prescription of (rightfully) de rigueur targeted therapy. Based on the data on LET/dose/CSC survival relationships, it is evident that HPT is a uniquely potent tool for CSC extirpation, that partial volume NIRT represents its maximum killing potency within safety limits, and that spatial fractionation may induce immunogenesis further augmented to abscopality by biologically guided partial volume selection and tightly constraining the PIM as an OAR.
Spatially fractionated and temporally ultrahypofractionated combination heavy ion therapy using the entire arsenal from helium to neon to ablate HRTVs defined by biological imaging as regions enriched with CSCs is the next logical progression following PATHY. As shown in SHArc and IMPACT Monte Carlo modeling, LET full-spectrum gradients are achievable and enable dose and/or LET de-escalation throughout the LRTV to mitigate the risk of compromising the PIM.25, 29, 42, 49, 50 In turn, LRTV sparing is the critical limiting step in determining dose and LET combinations necessary for achieving clinical CSC extinction.6, 25, 27 - 29, 32, 49 Moreover, only the delivery of such LET gradients may potentially extinguish the biological possibility of tumor recurrence through the combined effects of focal, direct CSC killing, and induction of bystander and immune secondary mechanisms transmitted over large relatively spared volumes.42, 46, 91 - 94, 108 - 118
Economics of HPT: Challenges of Upfront Capital Allocation, Potential for Long-Term Return, and Alternatives
A persistent perception that HPT facilities are prohibitively expensive to construct and operate remains a major barrier to the broader adoption, expansion, and patient access to this highly promising treatment modality. Though the capital requirements are undeniably high, the overall economics of HPT is more nuanced, and often more favorable, than commonly perceived. A comparative analysis of the cost-effectiveness of CIRT vs SBRT for the treatment of stage I non-small cell lung cancer at Gunma University found that the bulk of the difference was accounted for by costs of hospitalizations and ancillary studies rather than by technical fees for CIRT, the former of which could be mitigated or made more efficient.119 The same institution found the mean total cost of CIRT was lower than that of chemoembolization in the treatment of hepatocellular carcinoma (¥4,974,278 vs ¥5,284,524).120 A multi-institutional Japanese analysis found comparable total costs of treatment for locally recurrent rectal cancer using CIRT vs multimodality conventional treatment (¥4,803,946 vs ¥4,611,100).121 A multi-institutional European analysis found that the average cost per fraction delivered was €1128 at combined CIRT/PBT centers, €743 at PBT-only centers, and €233 at photon-only centers.122 These differences should be interpreted in light of HPT’s unique suitability for hypofractionation, owing to its biological insensitivity to fractionation, as well as the potential for HPT-driven improvements in local control to reduce health care costs.
Notably, capital costs and yearly operational costs for combined CIRT/PBT, PBT-only, and photon facilities were respectively found to be €138.6 million, €94.9 million, and €23.4 million, and €36.7 million, €24.9 million, and €9.6 million.122 These differences in initial and operational outlays are undoubtedly too much to bear for many health systems, even when factoring in cost-reducing public investment.123 The expansion of particle therapy capabilities to include oxygen, neon, and other heavy ion species not yet in routine clinical use is sure to exacerbate cost differences, at least in the short term. Both the public health benefit of improved oncologic outcomes and the potential for long-term cost savings support a strong case for increased public investment in HPT, as well as for international cooperation and cost-sharing to facilitate broader access.
However, strategies that enable functional delivery of high LET therapy to larger populations than can be served by HPT are in the early phases of clinical development and deserve recognition. A conjugate of the high LET α-particle emitting radionuclide actinium-225 (Ac-225) to the somatostatin receptor binding complex DOTATATE has been used in the early stage trials of gastrointestinal (GI) origin neuroendocrine tumors and metastatic paragangliomas, in the latter of which, in an admittedly small data set, the225 Ac-DOTATATE conjugate successfully controlled disease that had failed β-particle emitting lutetium-177 therapy.124 - 126
A clinical trial is underway investigating the225 Ac-DOTATATE conjugate for metastatic or unresectable somatostatin receptor-expressing breast cancers.127 Alpha emitters are a promising low-barrier means of delivering high LET therapy to appropriately selected patients, albeit limited anatomically to tumors to which they can be feasibly and reliably distributed, as well as far simpler than β emitters in terms of radiation safety precautions.
Another approach utilizes intratumoral infusion of nanoparticles composed of high Z materials with high electron density to increase the probability of ionization events for cells exposed to low LET radiation, functionally creating a field of high LET radiation delimited by the natural boundaries of gross tumor without risk of distribution to surrounding normal tissues. The phase II/III Act.In.Sarc. trial randomized 180 patients with locally advanced soft-tissue sarcoma indicated for neoadjuvant RT to intratumoral injection of the hafnium oxide-based NBTXR3 nanoparticle a week prior to RT to 50 Gy in 25 fractions followed in 5 weeks by surgical resection vs neoadjuvant monotherapy with RT and surgical resection after 5 weeks. The primary endpoint of pathologic complete response was doubled in the NBTXR3 arm over the RT-only arm (16% vs 8%) with all grade 3+ acute toxicity <10% and nearly identical rates of grade 3-4 wound complications.128, 129 The same agent has been incorporated into a pilot phase I trial for borderline resectable and unresectable non-metastatic PDAC, in which patients undergo intratumoral infusion of NBTXR3 prior to a 15-fraction course of RT.130 Finally, clinical exploration of boron neutron capture therapy, which uses intravenous administration of10 B 5 preferentially taken up by tumor cells then bombarded with slow neutrons to induce cell killing by high LET α-particles and lithium ions, is active and expanding.131 - 133
Strategies such as those just described cannot recapitulate all of the benefits and versatility of HPT, but are nevertheless a promising means of bridging the gap in access to offer some patients with aggressive/resistant cancers the opportunity for high LET therapeutic impact. These efforts should not be seen as exclusive of or in competition with HPT, but rather as complementary and potentially synergistic.
Conclusion
As discussed in Part I, high LET HPT has been shown in reproducible, basic research to be effective in the sterilization of CSCs. Consequently, it holds considerable promise as a means of significantly improving the prognostic paradigms of seemingly intractable human cancers whose outcomes are driven by their CSC biology. These findings are supported by early clinical studies demonstrating improved outcomes through crude deployment of HPT in the treatment of PDAC and GBM.14, 134 Moreover, the benefits of HPT appear to be synergistic with conventional and next-generation systemic therapies, though the ideal combinations and sequencing are yet to be determined. Economic and logistical challenges to expanding the reach of HPT are real but not insuperable, and the reduction it could entail in terms of reduced cancer recurrences may yield reductions in overall health care costs in the long run.
Literature to this point has downplayed the need for particles heavier than carbon ions to achieve true CSC eradication and overemphasized the so-called “overkill” problem. Safe implementation of oxygen or NIRT, however, necessitates selective targeting of high biological risk subvolumes within gross tumor to ensure critical OARs are not subjected to LET overdose. Multi-ionic LET painting models have been developed and validated as accomplishing this task. Likewise, clinically validated, state-of-the-art functional imaging technologies capable of detecting surrogates for CSC biology are already available, but have yet to be recognized for their potential utility in radiation oncology, let alone in defining HRTVs. Intriguingly, preclinical evidence suggests that selectively targeting CSC biology for high LET ablation may be the most promising approach to realizing the broader clinical potential of HPT, including the induction of immunogenic and abscopal cancer-killing effects. In this way, HPT may function not merely as a localized treatment but serve as an instrument of systemic cancer control.
Taken together, these advances suggest that the physics of high LET HPT may offer a radiobiological solution to the problem of CSCs and a tangible opportunity to alter the natural history of aggressive and treatment-refractory malignancies.135
References
Citation
Koffler DM, Ebner DK, Lehrer EJ, Fekrmandi F, Ehret F, Mahmoudi M, Beltran C, Trifiletti DM, Vallow L, Rutenberg MS, Eckstein J, Chitti B, Johnson B, Herman JM, Tinganelli W. Eradicating Cancer Stem Cells Using High Linear Energy Transfer Radiation Therapy Part 2: LET Painting and Other Advanced Techniques. Appl Radiat Oncol. 2025;(2):1 - 12.
doi:10.37549/ARO-D-25-00003P2
June 18, 2025