Breast MRI and its impact on partial breast irradiation
Images
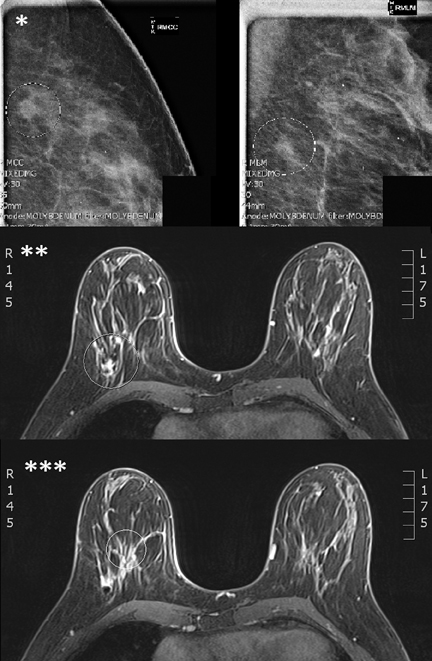
For women with early stage breast cancer, breast conserving surgery (BCS) followed by postoperative whole-breast irradiation (WBI)is associated with 85% to 95% long-term local control and is equivalent to a mastectomy in survival.1-4 The combination of BCS and adjuvant radiation is termed breast conservation therapy (BCT). The rationale for using WBI is to decrease local recurrence by eliminating potential small foci of tumors in the surgical bed or elsewhere in the breast. As 75% to 90% of recurrences occur at or near the surgical bed,5,6 an additional boost of radiation is delivered to the surgical bed following a moderate dose of WBI.
In the United States, WBI is typically delivered over 6 to 6 ½ weeks, 5 days per week. The time commitment for adjuvant radiation can be difficult for many women, particularly if they are not in close proximity to a radiation facility. This limited access to radiation facilities is one of the primary reasons why patients do not receive radiotherapy following BCS. Investigators have evaluated methods to shorten (ie, accelerate) therapy to increase the use of radiotherapy. The most common approach used to shorten therapy is accelerated partial breast irradiation (APBI). This approach delivers radiation only to the surgical bed, deliberately avoiding the rest of the breast.This drastically but safely shortens treatment from 6 weeks to 1 week or less.
APBI can be delivered via several different methods and is outside the scope of this article. The major risk of this approach is the small but real risk of a tumor recurrence 2 cm and further from the surgical bed. This concept is currently being tested nationally and internationally, with the largest protocol nearing completion through the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39. This study rapidly accrued patients in the most favorable population. Subsequently, the eligibility criteria were modified, and the study is currently open only to the high-risk population (ER-negative tumors, 1 to 3 involved lymph nodes, or young patients).
In 2009, based on published prospective and retrospective experiences, the American Society for Radiation Oncology (ASTRO) published consensus guidelines identifying patients that are “suitable,” “cautionary,” or “unsuitable” for APBI.7 A number of clinical and pathologic criteria were determined to be “suitable,” including patients aged ≥60, clinical unifocality with total tumor extent <2.0 cm (by mammography and ultrasound exams), tumor pathology of invasive ductal carcinoma or other favorable subtypes, and no lymph node or lymphovascular space involvement. All of the literature to date had been based on mammograms with or without an ultrasound.
Breast MRI has the highest sensitivity for detection of breast cancer (>90%). While mammography remains the gold standard for screening, breast MRI has been shown to identify mammographically and clinically occult breast cancer in certain subsets of patients.As a result, since 2007 the American Cancer Society has recommended breast MRI in combination with mammography for screening women who have a 20% to ≥25% lifetime risk of breast cancer.8 In a newly diagnosed breast cancer patient, breast MRI has been shown to assess tumor size more accurately than mammography and breast ultrasound. Additionally, breast MRI has shown higher sensitivity than conventional imaging for detection of multicentric and multifocal disease in the ipsilateral breast and in synchronous contralateral breast cancer. Additional multifocal or multicentric cancer is found by breast MRI in the same breast in 11% to 34% of women with unicentric breast cancer on conventional imaging,9,10 while synchronous contralateral breast cancer is found by MRI in 3% to 9% of patients.11 Even with all this supportive data, the role of breast MRI remains controversial as MRI has not been shown to reduce the re-excision rate or decrease local recurrences. One reason for this lack of benefit is the use of WBI, which is used to treat subclinical disease in the breast. With the advent of APBI, MRI may have a more significant impact as APBI deliberately avoids these uninvolved portions of the breast. This article seeks to review the literature related to the utility of MRI in the subset of patients considering treatment withAPBI.
The role of MRI in selecting candidates for APBI
Recently a number of studies have evaluated the ability of preoperative MRI to select patients for APBI. Godinez et al12 reviewed 79 patients who underwent preoperative bilateral breast MRI and were eligible for APBI. Patients were determined eligible preoperatively if they had lymph node negative, biopsy proven, unifocal invasive ductal carcinoma (IDC, 67 patients) and/or ductal carcinoma in situ(DCIS, 12 patients) ≤3.0 cm in greatest dimension by mammogram and ultrasound. The patients ranged in age from 29 to 75 years (mean 48). MRI identified a total of 80 additional lesions in the ipsilateral breast with 34 lesions in a different quadrant than the index cancer.
Thirty (38%) of the 79 patients were found to have additional biopsy-proven malignant tumors, of whom 8 had malignant foci outside of the quadrant in which the indexed lesion resided. Ultimately, only 62% of patients were considered appropriate for APBI.
An important critique of this study is the inclusion of a significant portion of young women and those considered high-risk due to a significant family history, a relative with the BRCA mutation, or a personal history of a BRCA mutation. Twenty-eight (35%) of the 79patients were <40 years old, and half were found to have additional malignant foci. Twenty-nine (37%) patients were considered high risk, and 41% had an additional malignant focus. It is unknown how many of these patients had the BRCA mutation. Per ASTRO guidelines, age <50 years or the presence of a BRCA mutation would make a patient “unsuitable” for APBI.
Tendulkar et al13 published a retrospective review of 260 patients who met criteria for the NSABP B-39/RTOG 0413 study, which are largely similar to the criteria that Godinez used. A significant difference in selection criteria was that invasive lobular carcinomas were included and 0 to 3 positive lymph nodes on final pathology were allowed in the Tendulkar study. All 260 patients had a bilateral breastMRI prior to surgery. Twenty-five percent of patients were <50 years old. There were 35 (13%) patients with ipsilateral suspicious findings by MRI, only 11 (4.2%) of which were proven to have multifocal/multicentric involvement. Thus, there was a 68.7% false positive rate. The median distance from the index lesion was 3 cm. There were 16 (6.0%) contralateral suspicious findings by MRI, 4 (1.5%) of which were biopsy-proven synchronous contralateral disease for a 75% false-positive rate. The authors notably report that multifocal ipsilateral invasive lobular carcinoma (ILC) was found in 3 of 17 (18%) cases, “significantly higher than that found in the aggregate of non-ILC histologies (3%, p = 0.04).” Current ASTRO recommendations for selection of candidates for APBI exclude the presence ofILC in consideration of this high rate of multifocality. Notably, Tendulkar et al did not find women younger than 50 to be at higher risk of synchronous lesions.
Most recently, Kuhr et al14 published a similar retrospective study of 113 patients with the exact criteria that Godinez used for selecting potential candidates for APBI. In 10 of 113 patients, MRI detected a total of 11 additional foci (7 ipsilateral, 4 contralateral), which were all found to be biopsy-proven cancers. Overall, MRI led to the detection of new ipsilateral and contralateral foci in 6.2% and 3.5%, respectively, of the patients initially considered candidates for APBI.
Compared to the Godinez study, in both the Tendulkar and Kuhr studies there was a far lower rate of detection of ipsilateral disease(38% versus 4% and 6.2%). There was also a smaller number of abnormal ipsilateral lesions on MRI in both the Tendulkar and Kuhrstudies compared to the Godinez studies, despite the much larger patient population (35 lesions in 260 patients and 7 lesions in 113 patients versus 80 lesions in 79 patients). Both the Godinez and Kuhr studies utilized 1.5 Tesla magnets, while Tendulkar used a 1.0 Teslamagnet. Although the differences observed in these studies may also be due to different patient populations, the discordance in the studies between the number of additional abnormal lesions identified in the Godinez study is significant and there does not appear to be a clear explanation for this. A recent meta-analysis demonstrated that MRI had a 67% positive predictive value for ipsilateral disease and37% for contralateral disease.15
Schmitz et al16 prospectively reviewed 62 women with pre-operative MRI imaging followed by wide local excision and histopathological correlation. There was excellent correlation between the index tumor and MRI-visible lesions with a mean size difference of 1.3mm. However, subclinical disease a distance of 1 cm or more from the MRI-identified tumor was identified in 52% of specimens, and subclinical disease a distance of 2 cm or more from the MRI identified tumor was identified in 25% of specimens.
MRI leads to the detection of a significant number of ipsilateral lesions in 4% to 38% of patients who are otherwise candidates forAPBI (Figure 1). Whether these lesions would develop into clinically significant breast cancers is unclear. Depending on the technique and extent of wide excision, some of these lesions may have been excised, and it is unclear whether APBI fields would cover these lesions. Additional data correlating imaging and pathology needs to be obtained in order to ensure an adequate margin of radiation treatment. It is in this area that further study needs to be done in regard to the utility of MRI in selecting patients for APBI.
The role of MRI in APBI planning
Previous data have suggested that computed tomography (CT) planning for APBI is suboptimal as it is often difficult to identify the lumpectomy site on CT imaging.17 One group found that MRI of the breast in the supine position yields a smaller, more accurately defined lumpectomy cavity with less interobserver variability than CT.18 However, Giezen et al19 found that the MRI did not add additional information to the surgical cavity delineation if the visualization score20 was low. Classically, WBI is performed in the supine position;however, with improved immobilization investigators can take advantage of the prone position, which displaces the surgical cavity away from various critical structures,21-23 making it easier to safely deliver the doses of radiation needed for APBI.
Ahn et al24 demonstrated the feasibility of using MRI guidance for planning of APBI in the prone position. Simulating 2 volunteers in both the supine (with both body and surface coils) and prone positions (with breast coils) demonstrated a clear superiority of the prone position by (1) reducing the signal-to-noise ratio (SNR), (2) reducing the deformation of the breast, and (3) reducing respiratory motion. The group also reported on the reproducibility of the setup.
Jozsef et al25 at New York University utilized cone-beam CT prior to performing APBI on 70 prone patients. They found the positioning to be reproducible with mean shifts of <0.2 cm in any direction.
MRI planning for APBI is both logistically feasible and reproducible and provides some clear advantages over CT planning particularly in visualization of the lumpectomy cavity. However, due to poor spatial resolution, CT is also needed to plan for accurate dose calculation and, subsequently, MRI and CT images will need to be fused. On-board imaging will require radiographic or CT anatomy to verify the treatment position. These uncertainties will need to be reduced further prior to the increased utilization of MRI.
The role of MRI in selecting patients for neoadjuvant radiation therapy to the breast
An interesting finding from NSABP B-39 has been that 3-dimensional conformal radiotherapy (3D-CRT), which treats the largest volume and has the shortest history of any technique, is by far the most commonly utilized delivery method utilized on this trial, encompassing >70% of the patients on the APBI arm. We suspect its popularity is related to the completely noninvasive nature of the approach compared to the 2 invasive brachytherapy approaches.
At the University of Maryland, we have previously investigated the potential benefits of delivering APBI using 3D-CRT in the preoperative setting and demonstrated that the radiated volumes are significantly smaller compared to those in the postoperative setting,increasing the number of patients eligible for partial breast radiation via this approach. In addition, the dose to all normal structures was also significantly reduced using preoperative APBI.26 This theoretical advantage could lead to improved cosmetic outcomes and decreased long-term toxicity, which has been seen in up to 10% of patients treated with 3D-CRT. Based on these advantages, we opened a feasibility study utilizing preoperative APBI-3D-CRT.
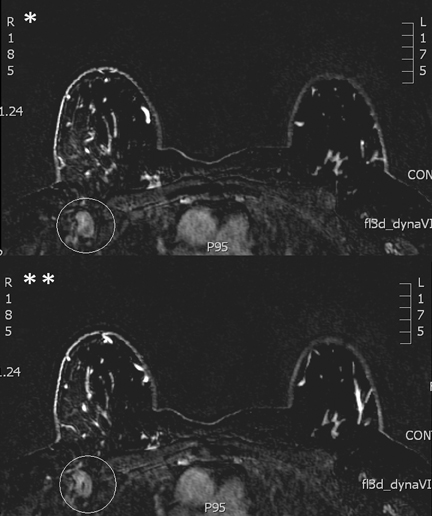
To be eligible for such treatment, patients not only have to meet the APBI criteria, but the risk of multifocal disease and nodal disease also needs to be excluded. To select potential candidates for neoadjuvant radiotherapy, MRI improves diagnostic accuracy of conventional imaging. As mentioned earlier, MRI finds ipsilateral mammographically-occult disease in 4% to 38% of patients who would otherwise be candidates for APBI. Further, breast MRI used with conventional imaging can also exclude axillary disease in all breast cancer patients with an estimated specificity of between 93% and 100% (Figure 2).27-29 Unfortunately, the sensitivity for staging the axilla is low. Thus, a woman with a clinical lymph node negative exam with an otherwise early stage cancer who underwent a breast MRI that is negative for additional foci of disease or for whom any additional MRI foci were demonstrated to be benign would be an ideal candidate for neoadjuvant radiotherapy.
MRI may also play a role in evaluating response to therapy similar to that seen following neoadjuvant chemotherapy. Based on the initial 12 patients treated with neoadjuvant APBI at UMMS, 25% had a complete pathologic response (pCR), which may increase with further escalation in dose, although developing noninvasive predictors of pCR is imperative before nonsurgical approaches can be considered. Functional MRI techniques can determine differences in vascular, biophysical, and biochemical responses in tumors versus the normal tissue.
Dynamic contrast-enhanced MRI30 is used to characterize vascular information in tumors based on the onset and rate of contrast enhancement to differentiate malignant from normal tissues. Diffusion-weighted MRI30-32 is used as a biophysical imaging marker to extract differences in the microenvironment between malignant and normal tissue based on the differences in rate of cellular growth, which is characterized using the diffusion coefficient of water. MR spectroscopy33-35 is used to measure the levels of different metabolites, such as choline, creatine, and lactate, in tissue, evidencing biochemical changes that occur in the tumor. Taken together, these 3 methods are likely to improve sensitivity and specificity in predicting treatment response, although further prospective data are warranted.
Conclusion
MRI improves preoperative loco-regional staging of breast cancer, which should translate into reducing the risk of occult multicentric disease in the breast. In addition, MRI adds another advantage for selecting patients for preoperative radiotherapy by accurately staging the axilla.
References
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002;347:1227-1232.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002;347:1233-1241.
- Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J. Clin. Oncol. 1998;16:441-452.
- van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European organization for research and treatment of cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-1150.
- Beitsch PD, Shaitelman SF, Vicini FA. Accelerated partial breast irradiation. J Surg Oncol. 2011;103:362-368.
- McCormick B. Partial breast radiation for early-stage breast cancer. Curr Opin Obstet Gynecol. 2012;24:31-37.
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from The American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol. Phys. 2009;74:987-1001.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Liberman, L, Morris EA, Dershaw DD, et al. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol. 2003;180:901-910.
- Morrow M, Freedman G. A clinical oncology perspective on the use of breast MR. Magn Reson Imaging Clin N Am. 2006;14:363-378.
- Lehman, CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295-1303.
- Godinez J, Gombos EC, Chikarmane SA, et al. Breast MRI in the evaluation of eligibility for accelerated partial breast irradiation. AJR Am J Roentgenol. 2008;191:272-277.
- Tendulkar RD, Chellman-Jeffers M, Rybicki LA, et al. Preoperative breast magnetic resonance imaging in early breast cancer: Implications for partial breast irradiation. Cancer. 2009;115:1621-1630.
- Kuhr M, Wolfgarten M, Stolzle M, et al. Potential impact of preoperative magnetic resonance imaging of the breast on patient selection for accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;81:541-546.
- Plana, MN, Carreira C, Muriel A, et al. Magnetic resonance imaging in the preoperative assessment of patients with primary breast cancer: systematic review of diagnosti caccuracy and meta-analysis. Eur Radiol. 2012;22:26-38.
- Schmitz AC, van den Bosch MA, Loo CE, et al. Precise correlation between MRI and histopathology - exploring treatment margins for MRI-guided localized breast cancer therapy. Radiother Oncol. 2010;97:225-232.
- Petersen RP, Truong PT, Kader HA, et al. Target volume delineation for partial breast radiotherapy planning: clinical characteristics associated with low interobserver concordance. Int J Radiat Oncol Biol. Phys. 2007;69:41-48.
- Jolicoeur M, Racine ML, Trop I, et al. Localization of the surgical bed using supine magnetic resonance and computed tomography scan fusion for planification of breast interstitial brachytherapy. Radiother Oncol. 2011;100:480-484.
- Giezen M, Kouwenhoven E, Scholten AN, et al. MRI- versus CT-based volume delineation of lumpectomy cavity in supine position in breast-conserving therapy: An exploratory study. Int J Radiat Oncol Biol Phys. 2012;82:1332-1340.
- Smitt MC, Birdwell RL, Goffinet DR. Breast electron boost planning: Comparison of CT and US. Radiology. 2001;219:203-206.
- Formenti SC, Truong MT, Goldberg JD, et al. Prone accelerated partial breast irradiation after breast-conserving surgery: Preliminary clinical results and dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;60:493-504.
- Jozsef G, Luxton G, Formenti SC. Application of radiosurgery principles to a target in the breast: A dosimetric study. Med Phys. 2000:27:1005-1010.
- Merchant TE, McCormick B. Prone position breast irradiation. Int J Radiat Oncol Biol Phys. 1994:30:197-203.
- Ahn KH, Hargreaves BA, Alley MT, et al. MRI guidance for accelerated partial breast irradiation in prone position: imaging protocol design and evaluation. Int J Radiat Oncol Biol Phys. 2009;75:285-293.
- Jozsef G, DeWyngaert JK, Becker SJ, et al. Prospective study of cone-beam computed tomography image-guided radiotherapy for prone accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;81:568-574.
- Nichols EM, Dhople AA, Mohiuddin MM, et al. Comparative analysis of the post-lumpectomy target volume versus the use of pre-lumpectomy tumor volume for early-stage breast cancer: Implications for the future. Int J Radiat Oncol Biol Phys. 2010;77:197-202.
- Garcia Fernández A, Fraile M, Giménez N, et al. Use of axillary ultrasound, ultrasound-fine needle aspiration biopsy and magnetic resonance imaging in the preoperative triage of breast cancer patients considered for sentinel node biopsy. Ultrasound Med Biol. 2011;37:16-22.
- Kvistad KA, Rydland J, Smethurst HB, et al. Axillary lymph node metastases in breast cancer: Preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol. 2000;10:1464-1471.
- Yoshimura G, Sakurai T, Oura S, et al. Evaluation of axillary lymph node status in breast cancer with MRI. Breast Cancer. 1999;6:249-258.
- Fangberget A, Nilsen LB, Hole KH, et al. Neoadjuvant chemotherapy in breast cancer-response evaluation and prediction of response to treatment using dynamic contrast-enhanced and diffusion-weighted MR imaging. Eur Radiol. 2011;21:1188-1199.
- Park SH, Moon WK, Cho, N, et al. Diffusion-weighted MR imaging: Pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology. 2010;257:56-63.
- Nilsen LB, Fangberget A, Geier, O, et al. Diffusion-weighted magnetic resonance imaging for pretreatment prediction and monitoring of treatment response of patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Acta Oncol. 2010;49:354-360.
- O’Flynn EA, Desouza NM. Functional magnetic resonance: Biomarkers of response in breast cancer. Breast Cancer Res.2011;13:204.
- Tozaki M, Oyama Y, Fukuma E. Preliminary study of early response to neoadjuvant chemotherapy after the first cycle in breast cancer: Comparison of 1H magnetic resonance spectroscopy with diffusion magnetic resonance imaging. Jpn J Radiol. 2010;28:101-109.
- Meisamy S, Bolon PJ, Baker EH, et al. Neoadjuvant chemotherapy of locally advanced breast cancer: Predicting response with in vivo (1)H MR spectroscopy—a pilot study at 4 T. Radiology. 2004;233:424-431.