Whole-Lung IMRT in Children and Adults With Synovial Sarcoma and Lung Metastases: Single-Institution Prospective Clinical Trial
Images
Abstract
Objective: To evaluate the toxicity and feasibility of whole-lung irradiation (WLI) in children and adult patients with synovial sarcoma and pulmonary metastases.
Methods and Materials: After completing standard therapy, 14 patients with synovial sarcoma and lung metastases (ages 12-52, mean 30 years) were treated with WLI in (n = 10) or as per (n = 4) a prospective trial with cardiac sparing intensity-modulated radiation therapy (IMRT) to 1500 cGy in 150 cGy per fraction. The primary objective was to assess the overall toxicity rate at 1 year after radiation, with a secondary objective to assess the pulmonary failure-free survival (PFFS).
>Results: Median follow-up among all patients was 33 months from time of IMRT (range, 3-69 months). At the time of IMRT, 13 of 14 patients had residual or recurrent gross disease in the lungs. At 18 months, the PFFS was 14.3%, with a median time to pulmonary failure of 6.2 months from IMRT. All acute toxicities from IMRT were grade 1, including fatigue (n = 9), esophagitis (n = 4) cough (n = 2), dermatitis (n = 2), nausea (n = 3), and dysphagia (n = 1). Late toxicities from IMRT at 1 year were minimal, including low-grade dyspnea and mild cough.
Conclusion: Whole-lung IMRT for patients with synovial sarcoma and lung metastases is feasible with minimal acute and late toxicity. However, long-term durable pulmonary control was not achieved in our cohort of patients with residual/recurrent gross pulmonary disease. Low-dose IMRT with 1500 cGy should be further explored as part of consolidation therapy (rather than in the setting of recurrent/residual disease) as is the standard for Ewing sarcoma and rhabdomyosarcoma.
Keywords: Synovial sarcoma, whole-lung irradiation, pulmonary metastases, consolidative therapy
Synovial sarcoma accounts for approximately 5% to 10% of all sarcomas1-3 and is frequently observed in young adults, with a mean age of 39.4 In addition, synovial sarcoma is the most common non-rhabdomyosarcoma sarcoma in children.5 Pulmonary metastases represent the most common site of metastases and is the leading cause of death in patients with synovial sarcoma.1,4
In patients with other radiosensitive pediatric sarcomas such as rhabdomyosarcoma (RMS) and Ewing sarcoma, whole-lung irradiation (WLI) is the standard of care as part of consolidation at the completion of planned therapy for patients with lung metastases.6-8
In this setting, WLI is well tolerated9 and associated with reduced pulmonary relapses and improved event-free survival (EFS).10 However, for patients with lung metastases and synovial sarcoma, WLI is not part of the treatment mainstay, despite the frequency of pulmonary metastases and potential oncologic benefit. Additionally, like RMS and Ewing sarcoma, synovial sarcoma is a radiosensi- tive histology.11,12 In this trial, we sought to evaluate the toxicity and clinical outcomes after cardiac-sparing, whole-lung intensity-modulated radiation therapy (IMRT) in patients with synovial sarcoma and lung metastases.
Methods
Patients
This was a single-institution prospective clinical trial of patients with synovial sarcoma and lung metastases at Memorial Sloan Kettering Cancer Center (MSKCC) treated between September 2014 and June 2022. Fourteen patients were treated with WLI in (n = 10) or as per (n = 4) the prospective trial. Patients completed standard therapy as determined by the primary management team (eg, surgery +/- radia- tion to the primary site and any adjuvant chemotherapy, most commonly Adriamycin + Ifosphamide + MESNA [AIM]) and were eligible for enrollment if they had lung metastases at diagnosis and/or developed lung metastases during the course of therapy. All patients had CT chest imaging prior to the start of WLI to serve as a baseline for follow-up scans and were recommended as per protocol to have
a baseline echocardiogram and pulmonary function tests (PFTs) prior to starting radiation treatment. The study was approved by the MSKCC Institutional Review Board/Privacy Board (IRB 14-075).
Radiation
All patients received cardiac-sparing IMRT to 1500 cGy in 10 fractions of 150 cGy per fraction, 1 fraction per day, in accordance with the protocol after metastatectomy and after or concurrent with chemotherapy. No patient received radiation therapy to the lungs prior to treatment. In general, patients were simulated in a supine position with an alpha cradle and without abdominal compression. The clinical target volume (CTV) was the bilateral lung volume, including all pleural recesses and bilateral hila. The internal target volume (ITV) included an expansion on the CTV to encompass the bilateral lungs on all phases of the respiratory cycle (as defined by the 4DCT). The planning target volume (PTV) was a 1-cm expansion in all directions on the ITV, to account for spatial uncertainties in patient positioning and treatment delivery. Further descriptions on the cardiac-sparing WLI and details on constraints can be found in previously published work.13 Three patients had an additional stereotactic body radiation therapy (SBRT) boost (25-30 Gy in 5 fractions) after WLI for treatment of gross disease.
Protocol Follow-up
Following completion of therapy, patients were to undergo CT chest imaging and toxicity assessments at 3, 6, 12, 18, and 24 months; an echocardiogram at 6, 12, and 24 months; and PFTs at 6 and 24 months.
Statistical Analysis
The primary objective of the study was to assess the safety of whole-lung IMRT following standard treatment in patients with synovial sarcoma and lung metastases. Secondary objectives were to determine rates of pulmonary failure-free survival (PFFS) and overall survival (OS) after completion of whole-lung IMRT. The safety endpoint included both acute (< 3 months from completion of WLI) and late toxicities (1 year from completion of WLI). The Common Terminology Criteria for Adverse Events, version 4.0, was used to grade acute and late toxicities. The PFFS was defined as survival with no progressive disease in the lungs from the initiation of IMRT, and OS was calculated as the time from initiation of IMRT to death, no matter the cause. Living patients at the time of analysis were censored at the time of the last follow-up visit. The Kaplan-Meier method was used to assess the PFFS and OS.
Results
Patient, Tumor, and Dosimetric Characteristics
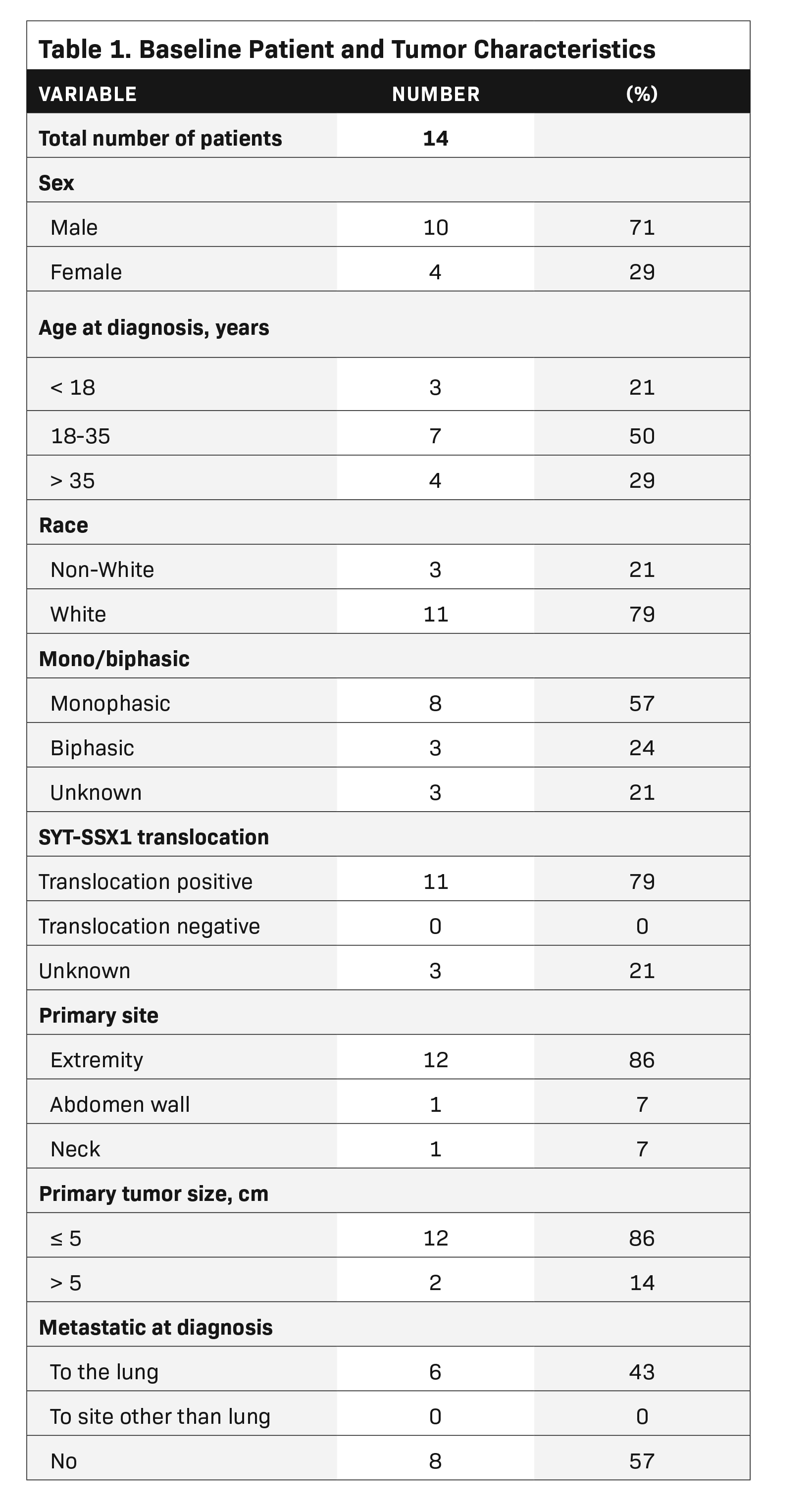
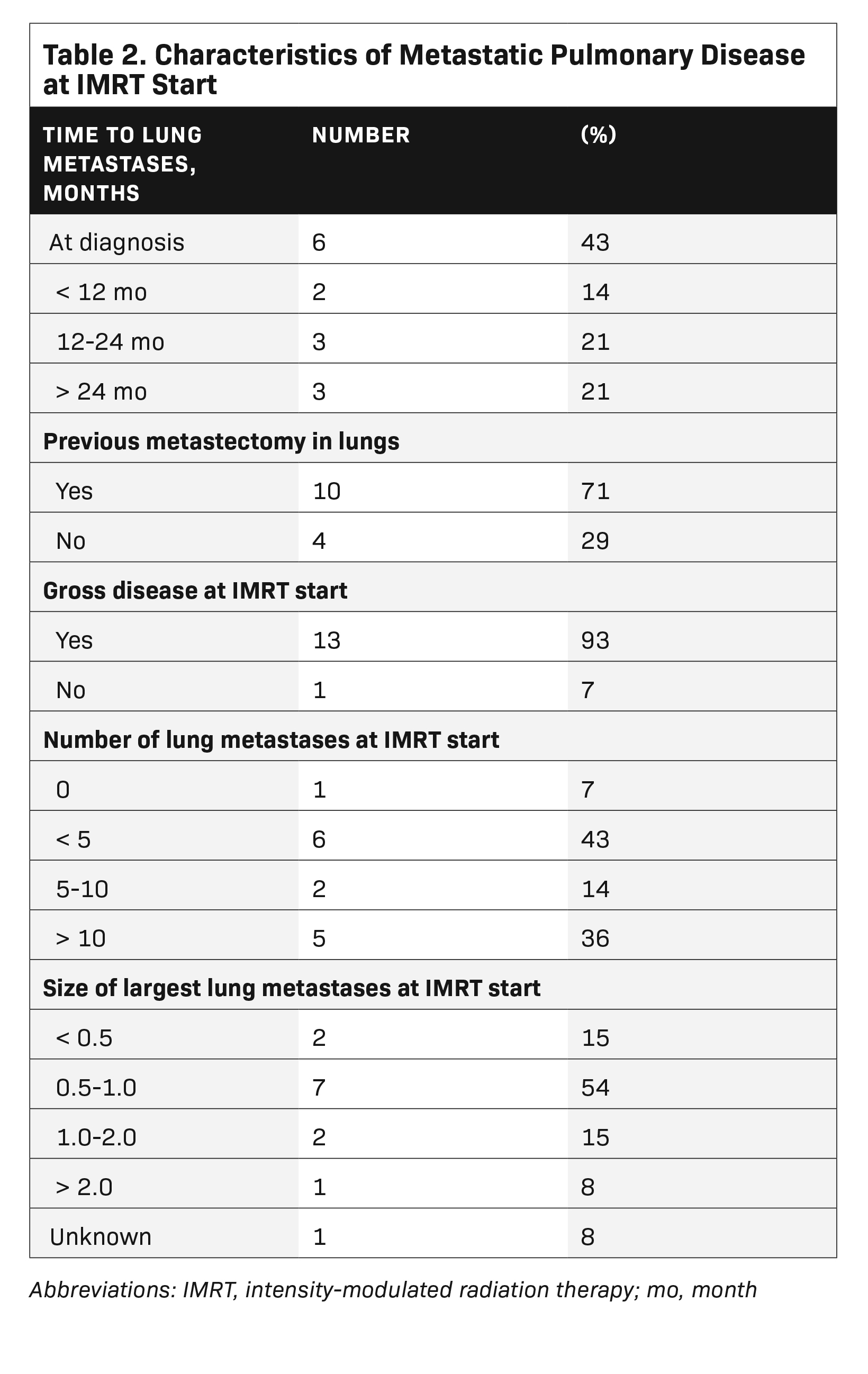
The median patient age at WLI was 38 years (range, 13-55 years), with 10 male patients and 4 female patients (Table 1). Six patients presented with lung metastases at diagnosis, while the other 8 patients developed lung metastases at a median time of 25 months from diagnosis (range, 9-39 months), after initial treatment failed (Table 2). All patients were treated with chemotherapy prior to or concurrent with lung RT. Ten patients underwent metastatectomies prior to the initiation of RT. Median follow-up among all patients was 33 months from time of IMRT (range, 3-69 months). At the time of WLI, 13 of 14 patients had residual or recurrent gross disease in the lungs, as determined by imaging prior to the start of RT. The median number of lung metastases at the start of RT was 4 (range, 1-10 metastases), with the average size of the largest metastasis being 1.0 cm (range, 0.3-3.2 cm). The average mean cardiac dose of all patients was 1058 cGy (range, 870-1286 cGy).
Clinical Outcomes
Twelve of 13 patients with pulmonary gross disease at the time of IMRT progressed at an initial pulmonary disease site after completion of IMRT. One patient never progressed in the lung after completion of chemotherapy and WLI, and has remained disease free for 30 months. The 1 patient with no gross disease at time of IMRT relapsed after 8 months at a new site of dis- ease in the lungs.
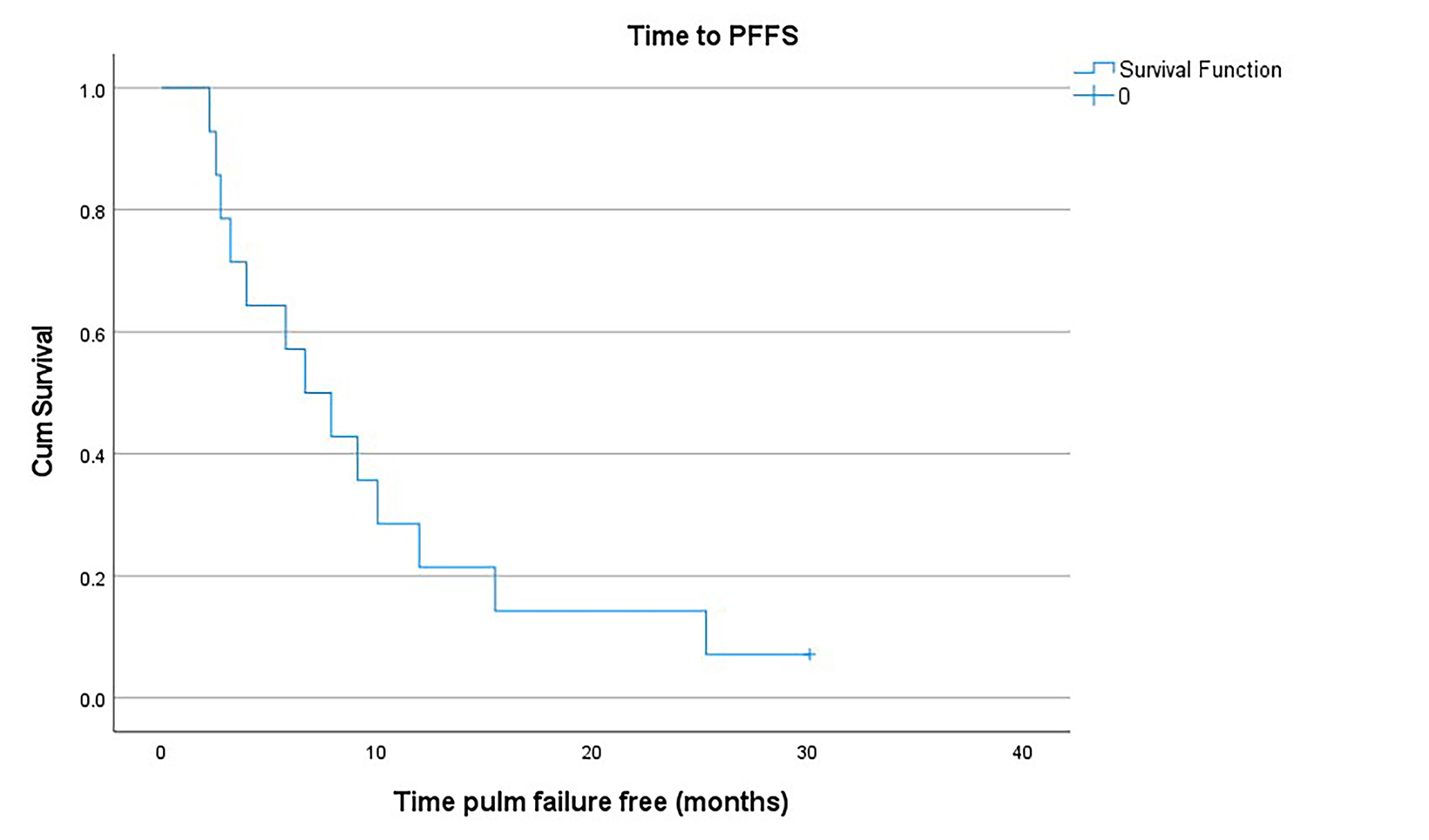
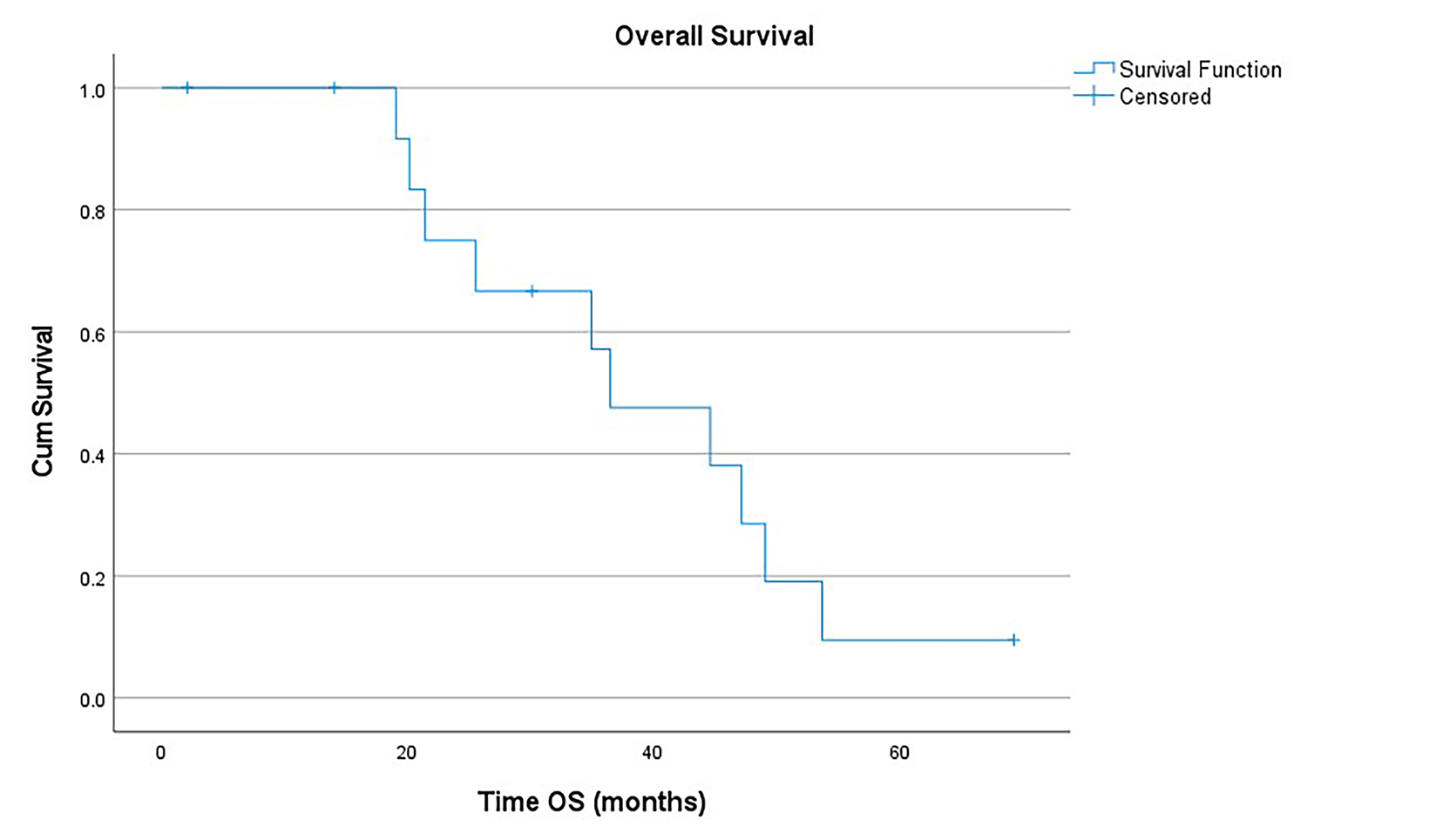
At 18 months, the PFFS was 14.3% (Figure 1). Two of 3 patients treated with SBRT for their pulmonary relapse experienced subsequent local pulmonary control. The OS at 18 and 36 months from IMRT was 100% and 57.1%, respectively (Figure 2). All patients had pulmonary disease at the time of death.
Toxicities
All acute toxicities from IMRT were grade 1, including fatigue (n = 9), esophagitis (n = 4) cough (n = 2), dermatitis (n = 2), nausea (n = 3), and dysphagia (n = 1). Late toxicities from IMRT at 1 year were minimal, including low-grade dyspnea and mild cough, although the etiology of these findings is likely multifactorial due to tumor burden, surgery, chemotherapy, and radiation therapy. No patients experienced an impairment in their daily functioning as a result of treatment. No significant decline in cardiac function as measured by echo (ejection fraction [EF] mean decline by 1.6%, P = 0.74), or pulmonary function as measured by PFTs (forced expiratory volume [FEV] mean decline 0.6%, P = 0.91; forced vital capacity [FVC] mean decline 1.0%, P = 0.99; diffusing capacity of the lungs for carbon monoxide [DLCO] mean decline 7.0%, P = 0.52) was seen at 1-year follow-up.
Discussion
Overall, whole-lung, cardiac-sparing IMRT is feasible for patients with synovial sarcoma metastatic to the lung and with minimal acute and late toxicities. However, in our patient cohort in which 93% of patients had residual gross disease in the lungs at the time of radiation, long-term pulmonary control was not achieved.
A dosimetry study comparing WLI using an anteroposterior-posteroanterior technique vs cardiac-sparing IMRT (CS-IMRT) showed the volume of the left ventricle, right ventricle, myocardium, and coronary arteries received a significantly lower radiation dose in the CS-IMRT plans as compared to the anteroposterior-posteroanterior plans.14 There has since been a multi-institutional protocol investigating the feasibility of cardiac-sparing whole-lung IMRT in children and young adults with a diagnosis of Wilms tumor, Ewing sarcoma or RMS, and lung metastases showing minimal long-term cardiac morbidity. In our study utilizing WLI IMRT for patients with synovial sarcoma, we were able to achieve a mean heart dose of 1058 cGy, and no difference in cardiac functioning as measured by echo was observed.
Regarding pulmonary toxicity following WLI delivered with IMRT, on a prospective trial including 20 patients with Wilms, RMS, and Ewing sarcoma, only 1 patient developed pulmonary restrictive disease.13 In studies that examine patients treated with low- dose WLI using conventional techniques, there are often mild reductions in pulmonary function abnormalities with low rates of clinically symptomatic moderate or severe pulmonary symptoms on follow-up.9,15,16 These results indicate that while pulmonary function test abnormalities are often seen after WLI, the incidence of clinically significant pulmonary toxicity is low, particularly at low doses of 15 Gy, the dose used in this study. In our study, there were no significant declines in cardiac or in pulmonary function as measured by PFTs, and no patients experienced toxicities impeding their daily activities at 1 year. Overall, WLI is widely tolerated among patients with synovial sarcoma as it is for patients with Ewing sarcoma and RMS.
Long-term durable pulmonary control was not achieved in our cohort of patients with residual/recurrent gross disease in the lungs at the time of radiation. The target population of this study was patients who initially presented with synovial sarcoma metastatic to the lungs, and who completed standard therapy without gross residual disease in the lungs at the time of radiation therapy. However, 8 of 14 patients had relapsed pulmonary disease during or after initial treatment, and 13 of 14 patients presented with gross residual disease in the lungs at the start of radiation therapy. Thus, our study included an unfavorable cohort of patients with progressive, bulky disease in the lungs at the time of WLI, unlike those typically treated with WLI as part of consolidation for
RMS and Ewing sarcoma. Furthermore, the majority of these patients did not receive a boost or additional treatment to their gross pulmonary disease.
WLI is considered standard at the end of therapy for patients with Ewing sarcoma and RMS with lung metastases. For both tumors, studies have shown an improvement in progression-free survival after consolidative WLI.6,9 Data indicate that local control of pulmonary metastases is associated with improved survival as well.10 Similar to RMS and Ewing sarcoma, synovial sarcoma is a radiosensitive histology11 that may benefit from such an approach. In addition, given the consistent pattern of pulmonary failure at a pre-existing site of gross disease, consideration of high-dose radiation such as SBRT after WLI to gross residual disease should be considered for optimal control of pulmonary metastases from synovial sarcoma, as is now done for patients with Ewing sarcoma and RMS.17 In our series, 2 of 3 patients treated with subsequent SBRT for pulmonary relapse obtained local pulmonary control. A series from the University of Rochester using SBRT for pulmonary metastases from soft-tissue sarcomas showed an 82% rate of local control at 3 years and an improvement in OS from 0.6 years to 2.1 years with the use of SBRT.18
Conclusion
In conclusion, our study shows that 15 Gy WLI with cardiac-sparing IMRT is feasible and well tolerated in patients with synovial sarcoma. However, this approach was not sufficient for treatment of patients with relapsed, gross residual disease in the lungs (13 of 14 patients included). Overall, we recommend that 15 Gy WLI with IMRT should be explored further (with consideration of an SBRT boost for gross disease) for patients with synovial sarcoma and lung metastases at the completion of initial therapy as part of consolidation therapy, rather than in the setting of recurrent/residual disease.
References
- Gazendam AM, Popovic S, Munir S, Parasu N, Wilson D, Ghert M. Synovial sarcoma: a clinical review. Curr Oncol. 2021; 28(3):1909-1920.
- Mastrangelo G, Coindre J-M, Ducimetiere F, et al. Incidence of soft tissue sarcoma and beyond: a population-based prospective study in 3 European regions. 2012;118(21)5339-5348.
- Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CDM, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epide- miology and end results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;19(12):2922-2930.
- Aytekin MN, Ozturk R, Amer K, Yapar A. Epidemiology, incidence, and survival of synovial sarcoma subtypes: SEER database analysis. J Orthop Surg. 2020;28(2):2309499020936009.
- McGrory JE, Pritchard DJ, Arndt CA, Nascimento AG, Remstein ED, Rowland CM. Nonrhabdomyosarcoma soft tissue sarcomas in children. The Mayo Clinic experience. Clin Orthop Relat Res. 2000;(374):247-258.
- Rodeberg D, Arndt C, Breneman J, et al. Characteristics and outcomes of rhabdomyosarcoma patients with isolated lung metastases from IRS-IV. J Pediatr Surg. 2005;40(1):256-262.
- Meisel JA, Guthrie KA, Breslow NE, Donaldson SS, Green DM. Significance and management of computed tomography detected pulmonary nodules: a report from the National Wilms Tumor Study Group. Int J Radiat Oncol Biol Phys. 1999;44(3):579-585.
- Dunst J, Schuck A. Role of radiotherapy in Ewing tumors. Pediatr Blood Cancer. 2004;42(5);465-470.
- Bolling T, Schuck A, Paulussen M, et al. Whole lung irradiation in patients with exclusively pulmonary metastases of Ewing tumors. Toxicity analysis and treatment results of the EICESS-92 trial. Strahlenther Onkol. 2008;184(4):193-197.
- Paulussen M, et al. Ewing’s tumors with primary lung metastases: survival analysis of 114 (European Intergroup) Cooperative Ewing’s Sarcoma Studies patients. J Clin Oncol. 1998;16(9):3044-3052.
- Wolden SL, Alektiar KM. Sarcomas across the age spectrum. Semin Radiat Oncol. 2010;20(1):45-51.
- Haas RL, Gloot BGJ, Scholten AN, et al. Cellular radiosensitivity of soft tissue sarcoma. Radiat Res. 2021;196(1):23-30.
- Kalapurakal JA, Gopalakrishnan M, Walterhouse DO, et al. Cardiac-sparing whole lung imrt in patients with pediatric tumors and lung metastasis: final report of a prospective multicenter clinical trial. Int J Radiat Oncol Biol Phys. 2019;103(1):28-37.
- Kalapurakal JA, Zhang Y, Kepka A, et al. Cardiac-sparing whole lung IMRT in children with lung metastasis. Int J Radiat Oncol Biol Phys. 2013;85(3):761-767.
- Weiner DJ, Maity A, Carlson CA, Ginsberg JP. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatr Blood Cancer. 2006;46(2):222-227.
- Ellis ER, Marchus RB Jr, Cicale MJ, et al. Pulmonary function tests after whole-lung irradiation and doxorubicin in patients with osteogenic sarcoma. J Clin Oncol. 1992;10(3):459-463.
- Kataria T, Janardhan N, Abhishek A, Sharan GK, Mitra S. Pulmonary metastasis from renal synovial sarcoma treated by stereotactic body radiotherapy: a case report and review of the literature. J Cancer Res Ther. 2010;6(1):75-79.
- Dhakal S, Corbin KS, Milano MT, et al. Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys. 2012;82(2):940-945.
Citation
Conte B, Casey DL, Wexler NKGH, Alektiar KM, Berry S, Wolden SL. Whole-Lung IMRT in Children and Adults With Synovial Sarcoma and Lung Metastases: Single-Institution Prospective Clinical Trial. Appl Radiat Oncol. 2023;(2):21-26.
July 4, 2023