Volumetric Changes in a Cervical Schwannoma in Response to Adjuvant Stereotactic Body Radiation Therapy: A Case Report
Affiliations
- 1 Department of Radiation Medicine, Roswell Park Comprehensive Cancer Center, Buffalo NY
- 2 Department of Biochemistry and Molecular Biology, Tulane University School of Medicine, New Orleans LA
- 3 Jacobs School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo NY
- 4 Department of Radiation Oncology, University of Rochester Medical Center, Rochester NY
- 5 Department of Neurosurgery,, State University of New York at Buffalo, Buffalo NY
- 6 Department of Neurosurgery, Roswell Park Comprehensive Cancer Center, Buffalo NY
Stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT) are the commonly employed treatment modalities for intra- and extracranial schwannomas. Transient swelling is common following SRS for vestibular schwannomas. We highlight the volumetric change following adjuvant SBRT of a schwannoma of the cervical spine. The patient initially presented with pain and numbness in the left arm, which led to diagnosis of a benign schwannoma in the cervical spine region. She then underwent subtotal surgical resection, followed by SBRT of the residual tumor. The volume of the schwannoma was measured on subsequent neuroimaging to ascertain the post-SBRT treatment response. To our knowledge, this is the first published report of transient swelling of a cervical schwannoma.
Case Summary
Schwannomas are rare tumors that arise from Schwann cells, which function to myelinate the peripheral nervous system. Within this benign entity, cervical schwannomas account for just 0.1% of all diagnoses.1 The preferred treatment for cervical schwannomas entails total tumor resection; however, obtaining clear margins may not be feasible in some patients owing to the proximity of nearby critical structures.2 - 4 Adjuvant radiation therapy is considered for positive margins or gross residual disease.5
Stereotactic body radiation therapy (SBRT) delivers a highly conformal tumoricidal dose while minimizing radiation exposure to the surrounding tissues.6 The steep dose fall-off achieved by SBRT is paramount in cases where the tumor is close to critical structures or vasculature, as is common in cervical schwannomas. The post-stereotactic radiosurgery (SRS) response of vestibular schwannomas (historically termed acoustic neuromas) is well documented in the literature, as cranial nerve VIII is the most common site of schwannoma development.7 These tumors tend to expand after SRS, which can be misinterpreted as tumor progression.8 - 12 Regardless of actual or pseudoprogression, volume expansion following SRS poses a threat to critical structures. Indeed, tumor expansion may cause temporary or permanent hearing loss, gait imbalance, facial twitching, palsy or sensory changes from impingement of inflammation of cranial nerves V and VII; hydrocephalus from the 4th ventricle obstruction; or brainstem injury.13 By extrapolation, when irradiating extracranial schwannomas with SBRT, radiation oncologists must be cognizant of the potential volumetric changes post-SBRT treatment. However, a paucity of literature describes post-SBRT changes in cervical schwannomas. We describe here for the first time, to the best of our knowledge, volumetric changes in a cervical schwannoma following adjuvant SBRT.
Presentation
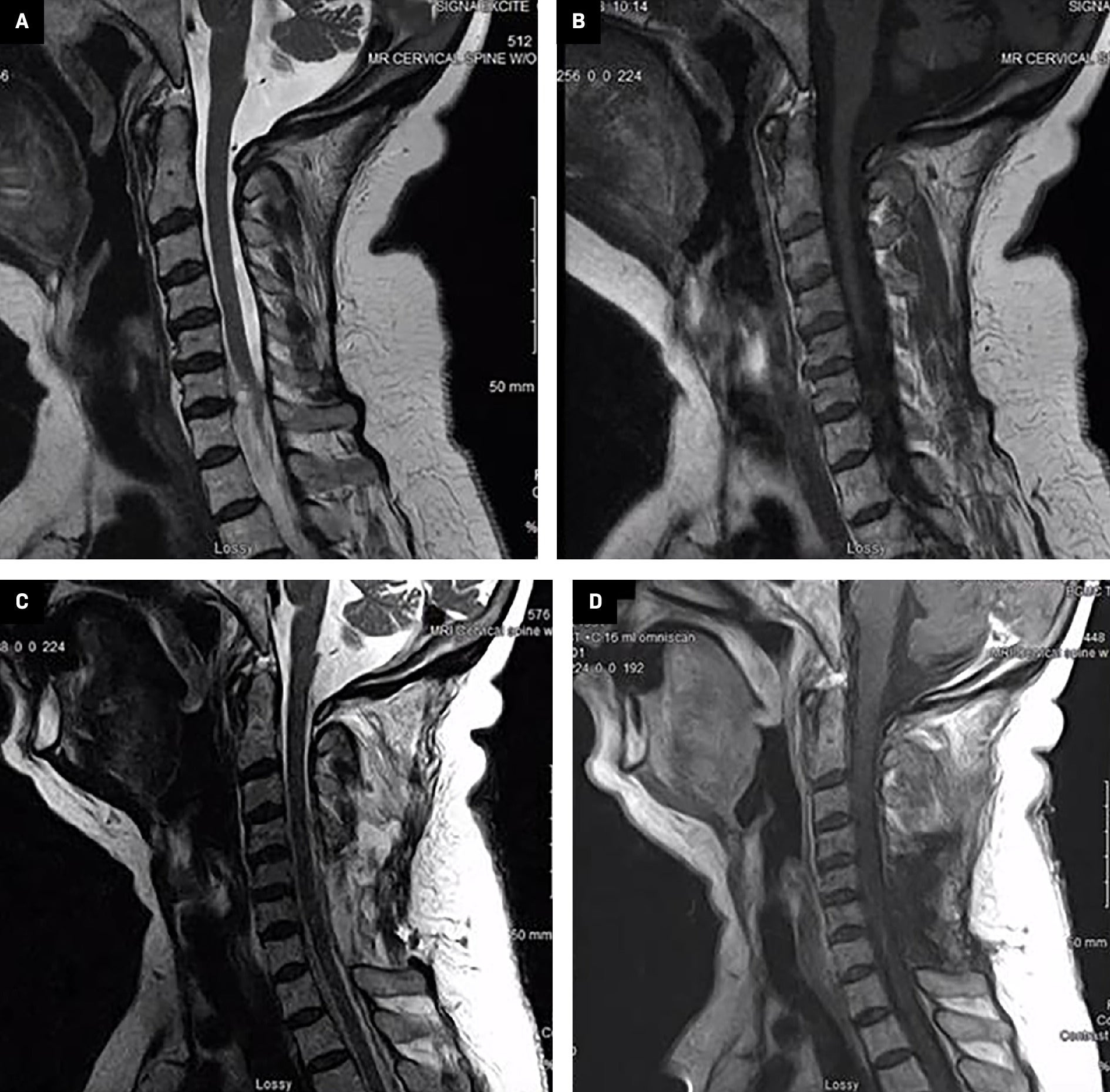
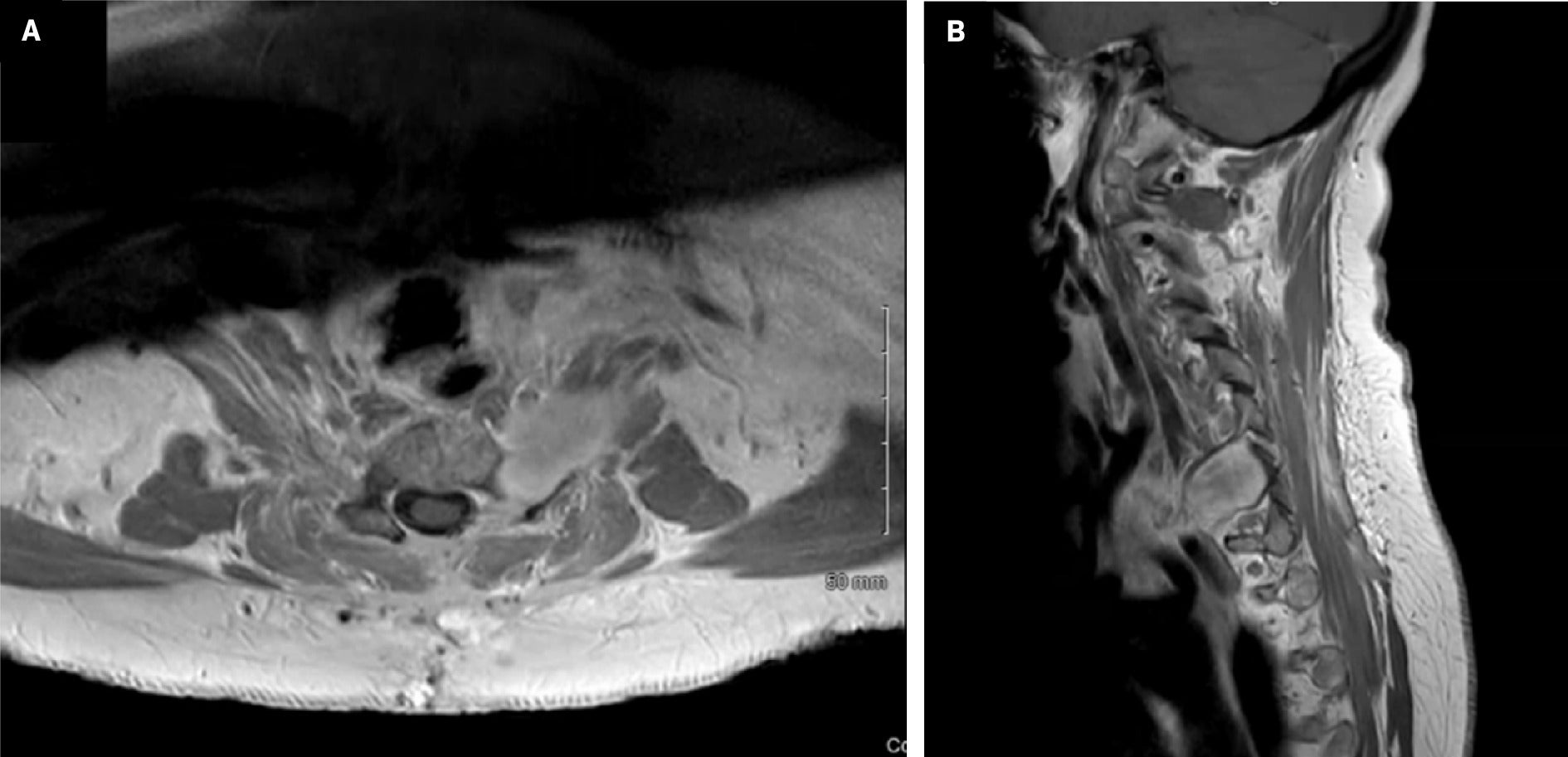
The patient was a 60-year-old female non-smoker with a history of left breast mastectomy for stage 3A breast cancer 11 years earlier and left shoulder replacement 7 years earlier. She initially presented with pain involving the left shoulder and arm and described the pain as a sharp intermittent sensation with no exacerbating factors. The pain was initially attributed to a combination of left shoulder replacement and lymphedema in the left arm following breast cancer treatment. However, the pain persisted, and the patient underwent a contrast-enhanced cervical MRI, which revealed a nerve sheath tumor in the C6-C7 region, extending extracanalicular and into the canal with some spinal cord compression ( Figure 1 ). She underwent a left partial C6 and C7 schwannoma resection with hemilaminectomy and posterior C4-T2 fusion for postlaminectomy kyphosis. Surgical intervention resulted in moderate pain relief. Pathology showed a myxoid peripheral nerve sheath tumor, with S100 protein diffusely positive and MIB-1 estimated to be 3%. A surveillance MRI ~7 months postsurgery revealed a residual tumor with a volume of 9.47 cm3 in the anterior region near the brachial plexus ( Figure 2 ). The patient was followed by neurosurgery and referred to radiation oncology 11 months postresection.
Contrast- and noncontrast-enhanced MRI of the cervical spine demonstrating preoperative cervical schwannoma (top) and postoperative surgical cavity (bottom). (A) Preoperative sagittal T2, (B) preoperative sagittal T1 sequence with contrast, ( C ) postoperative sagittal T2, and (D) postoperative sagittal T1 sequence with contrast.
Contrast-enhanced MRI of the cervical spine demonstrating a postoperative residual tumor in the anterior region adjacent to the brachial plexus. ( A ) Axial T1 sequence with contrast; ( B ) sagittal T1 sequence with contrast.
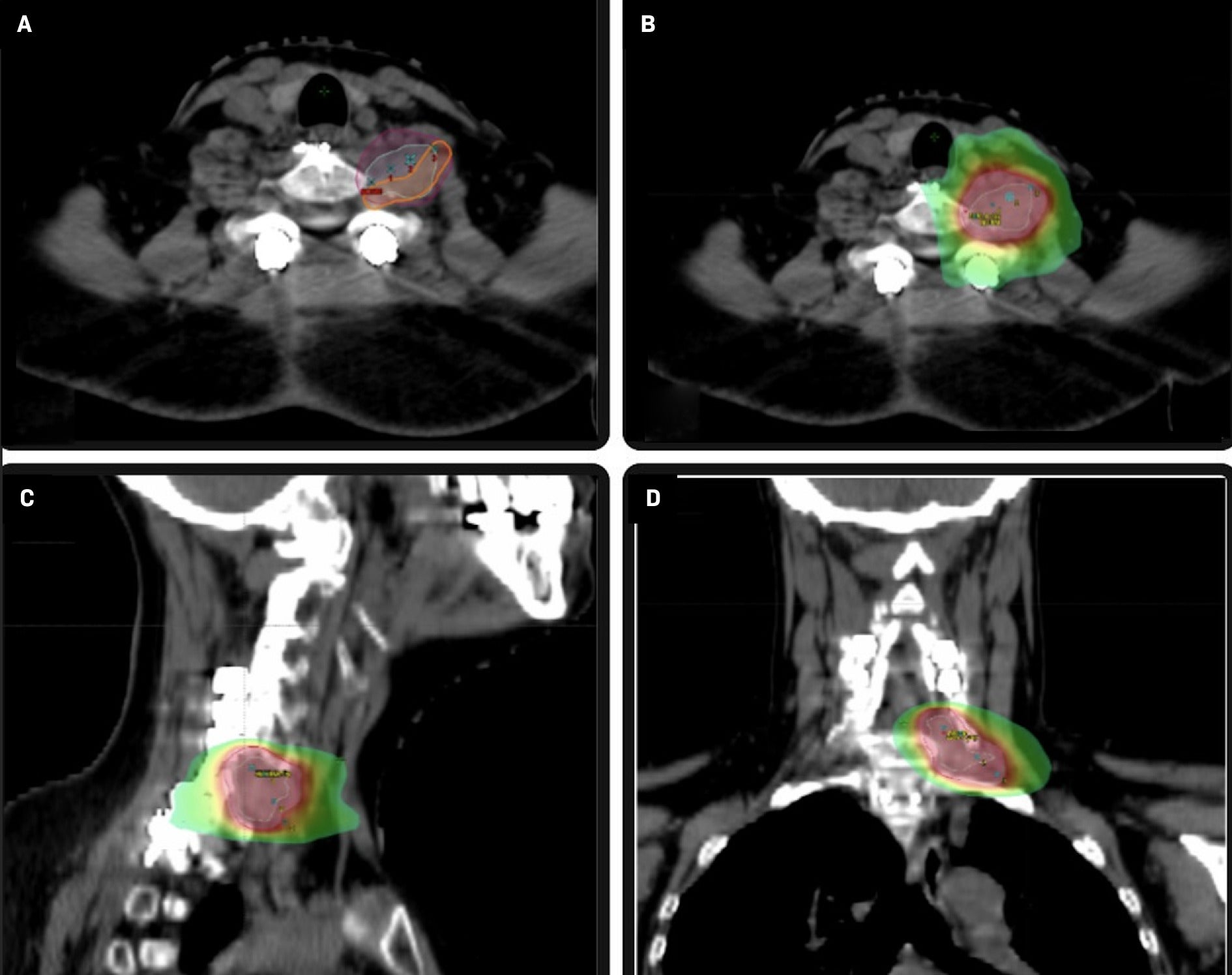
Eleven months following resection, the patient underwent SBRT with a dose of 2100 cGy in 3 fractions delivered every other day over a 5-day period. The patient was simulated and treated with an aquaplast mask on an Accufix board indexed to the table for immobilization. The gross tumor volume (GTV) was contoured using the planning CT scan and fused volumetric MR images. The planning target volume (PTV) was defined as a 5 mm circumferential expansion of the GTV. A volumetric modulated arc therapy plan was generated with 3 coplanar 6 MV flattening filter-free beams. The plan was normalized such that the prescription dose covered 95% of the PTV. The maximum PTV dose was 2363.5 cGy( Figure 3 ). The patient was treated on a Varian TrueBeam linear accelerator. Serial MRI was utilized to monitor tumor response to SBRT. Volumetric changes were calculated retrospectively from MRI by importing the MRI into the treatment planning system to contour the post-SBRT residual tumor and compute volumes.
Axial CT images ( A ) illustrating gross tumor volume 2100 (cyan), planning target volume (PTV) 2100 (magenta), and brachial plexus contour (orange). Stereotactic body radiation therapy axial ( B ), sagittal ( C ), and coronal planes ( D ) illustraing dose color wash (PTV 2100 shown in magenta).
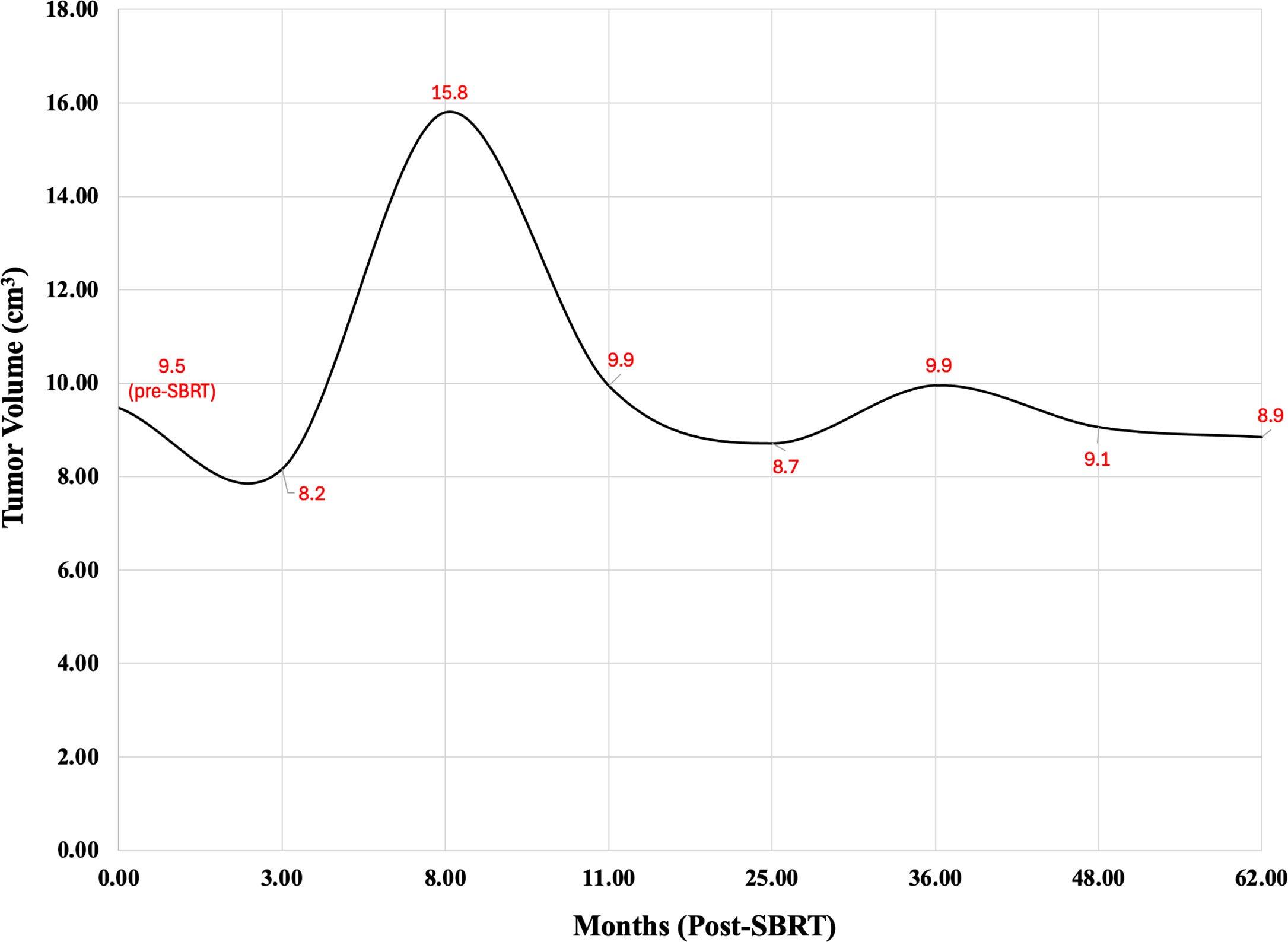
Three months following SBRT, MRI revealed that the tumor initially shrank to 8.16 cm3 . However, this regression in size was short-lived, and a scan 5 months later (8 months post-SBRT) demonstrated that the mass had grown to 15.8 cm3 . Three months later (11 months post-SBRT), the tumor had shrunk to 9.94 cm3 . Fourteen months after SBRT, the patient complained of increasing numbness in her left hand, but an MRI scan at that time revealed that the tumor had continued to shrink steadily. She described numbness in the left middle finger with occasional involvement of the 1st and 2nd digits but reported no upper extremity weakness or other neurologic symptoms. The tumor continued to shrink, stabilizing to a final volume of 8.85 cm3 > 5 years after SBRT. The patient reported persistent tingling and numbness along the left middle finger at the latest follow-up.
In total, tumor volume was obtained via cervical MRI 8 times—the first of which followed surgical resection just before radiation therapy. Tumor volumes at various periods are shown in Table 1 and Figure 4 . The patient was last seen in a follow-up 5 years after completing SBRT.
MRI-Determined Tumor Volume (cm3 ) Before and After Stereotactic Body Radiation Therapy (SBRT) Treatment on 9/2019
| MRI Date | Tumor Volume (cm3 ) |
|---|---|
| Pre-SBRT | 9.5 |
| 3 months post-SBRT | 8.2 |
| 8 months post-SBRT | 15.8 |
| 11 months post-SBRT | 9.9 |
| 25 months post-SBRT | 8.7 |
| 3 years post-SBRT | 9.9 |
| 4 years post-SBRT | 9.1 |
| >5 years post-SBRT | 8.9 |
Graphical depiction of tumor volume over time. SBRT, stereotactic body radiation therapy.
Discussion
As schwannomas are extremely rare in the cervical spine,1 the majority of data describe vestibular schwannoma treatment. SRS is often employed nonoperatively to achieve local control of vestibular schwannomas14 ; the high doses and steep dose falloffs offer local control rates of up to 90% while minimizing damage to the surrounding structures.15 Tumor expansion peaks approximately 6-12 months after SRS and can generally be attributed to pseudo-progression; this postradiotherapeutic tumor change is not indicative of treatment failure.9 Our case highlights the utility of SBRT to target the residual lesion adjacent to the brachial plexus. Using adjuvant stereotactic radiation therapy for vestibular schwannomas, Dhayalan et al reported a local control rate of 77.3%.16 In the spine, adjuvant SBRT has been shown to achieve successful local control of S1 nerve root melanotic schwannomas5 and benign thoracic spine schwannomas.17 However, the effect of adjuvant SBRT on benign schwannomas in the cervical region is not well documented.
This is the first report on volumetric changes of a cervical schwannoma following adjuvant SBRT. The tumor swelled approximately two-thirds of its pre-SBRT size ~8 months following treatment. After this initial expansion, the size steadily decreased and was slightly smaller than its pre-SBRT size 48 months after treatment.
In contrast to cervical schwannomas, characteristic volumetric changes following SRS are well documented for vestibular schwannomas. Meijer et al reported that 11 of 45 (24%) treated tumors initially increased in volume and eventually decreased to below pretreatment volume.18 The mean increase in volume was reported to be 25% after a mean follow-up time of 15 months. The tumors then shrank eventually to a volume lower than the pretreatment volume at an average of 34 months. Mohammed et al described 7 of 18 (39%) vestibular schwannomas that demonstrated pseudoprogression and then shrank below their pre-SRS volume.11 They reported a mean tumor volume increase of 35% and an average time to regression of 24 months.11 In contrast, the tumor in our case demonstrated a 67% increase in volume, which is markedly higher than the averages reported by these studies.
The patient was noted on the last follow-up to have persistent left middle finger numbness and tingling. We postulate that these symptoms could likely be the result of a late brachial plexopathy or neuropathy from the nerve root. Upon review, the dose to the brachial plexus was 2337.3 cGy (max dose per Dose Volume Histogram), which met the constraint of D0.03cc < 26 Gy, but the partial volume of 8.50 cc exceeded D3cc < 22 Gy, per the BR002 trial.19 Prior studies have demonstrated that brachial plexus volume exposure may be more critical than the maximum dose in terms of symptomatic motor or sensory deficits of the upper extremity.
Conclusion
Unlike the high number of studies regarding vestibular schwannomas, there is a significant dearth of data regarding the post-SBRT expansion of schwannomas in other areas, with little to no reports of volume expansion or percent changes in volume. To better understand the risks of complications following SBRT, the dynamics of tumor volumes of schwannomas in all locations should be investigated in greater depth. The result of such investigations would allow radiation oncologists and patients to make more educated decisions regarding the use of radiation therapy.
References
Citation
ND A, TV S, J R, Shekher,R, Madhugiri, V, V G, MT M, EI L, Prasad, D. Volumetric Changes in a Cervical Schwannoma in Response to Adjuvant Stereotactic Body Radiation Therapy: A Case Report. Appl Radiat Oncol. 2025;(1):37-41.
doi:10.37549/ARO-D-25-00005
March 1, 2025