Robotic-Assisted Seminal Vesicle Excision vs Brachytherapy for Isolated Seminal Vesicle Recurrence: 2 Case Reports
Affiliations
- 1 Department of Radiation Oncology, Kaiser Permanente, Los Angeles Medical Center, Los Angeles, CA
- 2 Department of Urology, Kaiser Permanente, Los Angeles Medical Center, Los Angeles, CA
The most common treatment for isolated seminal vesicle recurrence (ISVR) has been androgen deprivation therapy (ADT). Prior to the introduction of prostate-specific membrane antigen PET (PSMA-PET), detecting ISVR was imprecise, with most patients treated with ADT in the setting of biochemical failure. However, with the advent of high-sensitivity and -specificity PSMA-PET, identifying patients with ISVR is now possible, and local therapy may potentially offer cure with acceptable morbidity. We present 2 cases of ISVR treated with robotic-assisted seminal vesicle excision (RASVE) vs low-dose-rate (LDR) salvage brachytherapy, both of which rendered the patients disease free with undetectable prostate-specific antigen with short-term follow-up. RASVE and LDR salvage brachytherapy are reasonable treatment options for ISVR, with curative intent.
Keywords: brachytherapy, robotic-assisted seminal vesicle excision, isolated seminal vesicle recurrence, prostate cancer
Case Summary
Case 1 was a 62-year-old patient with a T1c Gleason score (GS) of 4+3, initial PSA 12.1, +5/12 cores. The patient underwent low-dose-rate (LDR) brachytherapy in 2014 using 114 loose iodine-125 seeds, 0.414 milliCuries per seed, to a minimum peripheral dose (MPD) of 14,400 cGy. He developed biochemical failure in 2017, with a prostate-specific antigen (PSA) score of 3.9. MRI and prostate biopsies were negative. The PSA continued to rise to 8.8 in 2018, and Axumin-PET demonstrated uptake in multiple subcentimeter periaortic lymph nodes, with a maximum standardized uptake value (SUV) of 4.5. In 2019, the patient was started on leuprolide and administered nodal radiation to 4000/200 cGy, but his PSA rose to 2.3 with a testosterone level of 60 in 2024.
Case 2 was a 68-year-old patient with cT2a, GS 6, and iPSA 16.0 who underwent brachytherapy in 2016 using 93 loose iodine-125 seeds to an MPD of 14,400 cGy. He developed biochemical failure with a PSA of 4.8 in 2024, and prostate biopsies were negative.
Diagnosis and Treatment
For case 1, PSMA-PET had an SUV of 15 at the left seminal vesicle (SV) ( Figure 1 ) in 2024, when his PSA was 2.3 with a testosterone level near castrate. Uronav (Invivo Corp) MRI fusion biopsy showed GS 4+3 in 5 cores with 70% involvement of the left SV, but prostate biopsies were negative. The patient subsequently underwent robotic-assisted seminal vesicle excision (RASVE) with extended pelvic lymph node dissection, in which bilateral SV and the adjacent prostate base were resected en bloc. Pathology demonstrated GS 3+4 involving bilateral SV with extension into peri-SV soft tissue, with negative deep margins and negative nodes (0/13). The patient’s PSA was undetectable 4 months post-surgery with no side effects or complications. In retrospect, we felt that his prior Axumin-PET, which showed positive nodal disease, likely represented a false-positive scan, as PSMA-PET is now considered more accurate.
Prostate-specific nembrane antigen PET for case 1 showing abnormal uptake left seminal vesicle.
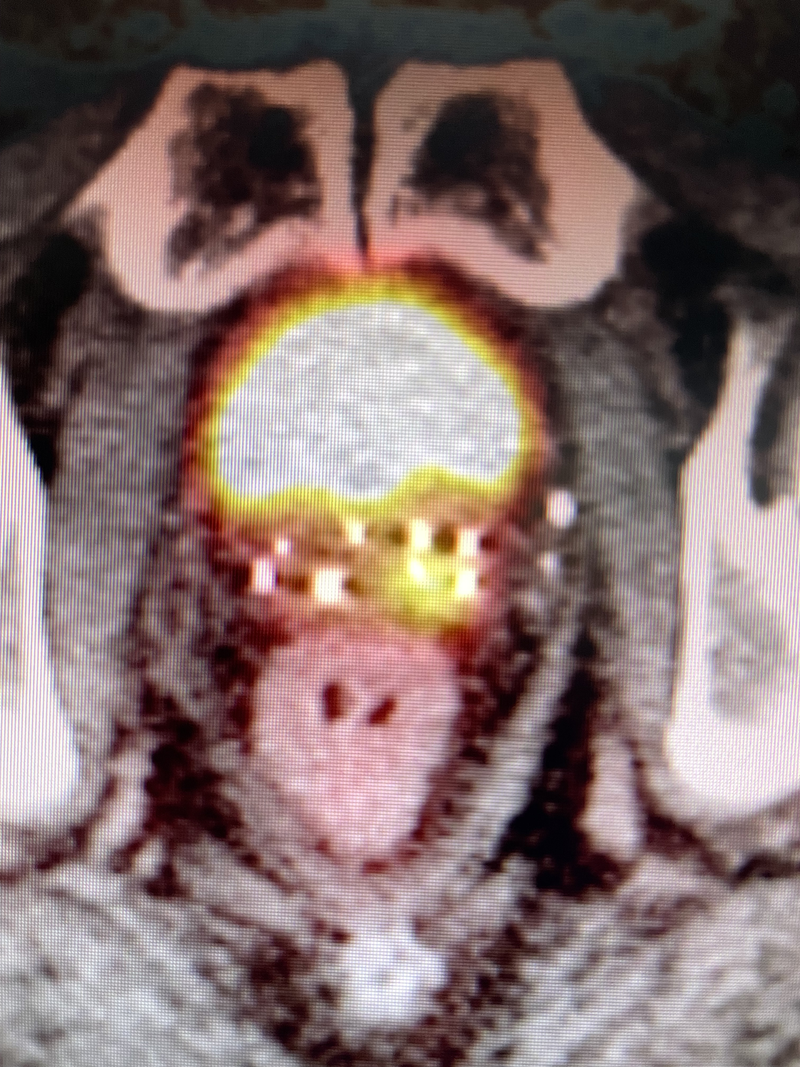
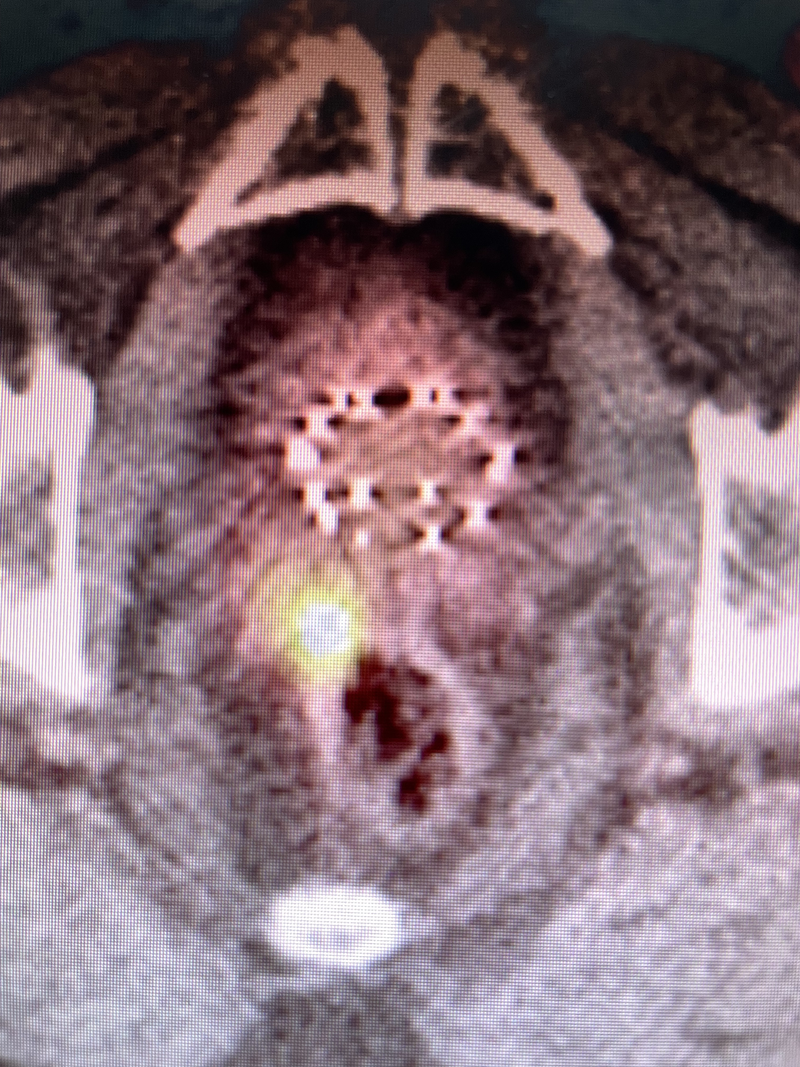
For case 2, the patient’s initial MRI in 2016 showed diffusion restriction and enhancement of an anterior midline lesion at mid-gland, an adjacent anterior lesion just left of the midline anterior lesion as well as a third lesion at the posterior right apex, but the SVs were normal. After brachytherapy in 2016 ( Figure 2 a), he developed biochemical failure in 2022 with a PSA of 4.8, but his sextant prostate biopsies were negative. PSMA-PET in 2024 showed recurrence in the right medial SV (SUV 16.7, Figure 3 ). This patient then underwent salvage brachytherapy with rectal spacer placement (Spacer OAR, Boston Scientific) as this was salvage treatment with previous radiation. We used 3 strands on the right SV, 2 of which had 5 seeds, while the third strand contained 6 seeds in the middle of the abnormal PSMA uptake. Also, 2 strands using 4 seeds each were placed in the left SV, for a total of 24 seeds with an activity of 0.382 milliCuries per seed using stranded iodine-125 ( Figure 2 b). Our prescription was 14,400 cGy to the entire right SV and proximal-mid-left SV. Interestingly, Figure 2B demonstrates a significant decrease in the size of the prostate over 8 years compared with Figure 2A , while Figure 2B contains 24 additional seeds to the SV. Androgen deprivation therapy (ADT) was not used for this patient, whose PSA 12 months post-implant was <0.1 without any complications from his salvage brachytherapy/SV implant.
Brachytherapy implant for case 2 for definitive treatment in 2016 on the left (A), while on the right (B) showing brachytherapy implant of seminal vesicles in 2024 in addition to patient’s seeds from 2016.

Prostate-specific membrane antigen PET for case 2 showing abnormal uptake right seminal vesicle.
Discussion
Historically, SV failure has portended systemic disease, and patients were usually treated with ADT, owing to suboptimal assessment of isolated seminal vesicle recurrence (ISVR). With the advent of PSMA-PET, finding patients with a locally recurrent, curable disease is now possible. Robotic-assisted salvage prostatectomy has been performed on patients developing local recurrence after external radiation and/or brachytherapy, although this is done less commonly due to its high complication rate. For special cases of ISVR, RASVE may be used, with cure rates comparable to salvage prostatectomy but with the added benefit of lower morbidity.
The largest series of RASVE reported on 17 patients, with a positive margin rate of 41% and a 3-year failure-free survival of 53%.1 In this series, 71% had bilateral SV involvement pathologically. Pretreatment biopsies showed only 35% with bilateral disease, while MRI demonstrated 12% with bilateral disease, and PSMA-PET showed 6% with bilateral disease. Thus, during any treatment of ISVR, we recommend treating both SVs, as was the situation in case 1 pathologically, where preclinical assessment only demonstrated unilateral disease. The treatment of ISVR using salvage brachytherapy has been described in only a few case reports of patients treated with SV recurrence with or without prostate recurrence.2, 3 With the advent of reliable stranded seeds, implantation in the SV is now possible, whereas previously, loose seeds would not stick to the SV owing to its spongy consistency.
Our institutional treatment preference for ISVR is RASVE as it offers pathologic staging with nodal assessment, and its side effects seem acceptable for either approach. One may hypothesize that salvage brachytherapy can be performed as a first salvage and, if unsuccessful, followed by RASVE as a backup treatment. However, this approach could increase complications as excessive radiation to the uretero-vesicle junction may lead to devascularization after RASVE if extracapsular techniques of brachytherapy are used initially.4
Our recommendation is to offer salvage brachytherapy to patients considered medically inoperable or to those who decline RASVE. Owing to the small number of patients with ISVR treated with RASVE or salvage brachytherapy, however, applying both these techniques to other institutions may be challenging as they may not be offered at most institutions, and most patients historically have been treated with ADT on an indefinite basis.
Our sample size is small, with only a short-term follow-up. However, in our experience, when patients reach a PSA of <0.1 after brachytherapy alone without ADT, the long-term cure rates are extremely high in the setting of de novo disease (unpublished data). There has been a significant decline in the number of institutions offering standard LDR brachytherapy,5 - 7 and even fewer offer more advanced extracapsular prostate brachytherapy, which can be used to treat unfavorable intermediate- and high-risk prostate cancer with brachytherapy alone.4, 8 - 10 We have implanted the SV and the prostate with the goal of reducing future risk of ISVR.4 Thus, we describe here a case where LDR salvage brachytherapy can be used to treat ISVR. Nevertheless, finding physicians technically able to perform the procedure is challenging as even standard LDR brachytherapy for prostate cancer may become obsolete due to low reimbursements.
Conclusion
RASVE and LDR salvage brachytherapy are reasonable treatment options for ISVR, with possible curative results, sparing some patients from long-term ADT.
References
Citation
DS GBF. Robotic-Assisted Seminal Vesicle Excision vs Brachytherapy for Isolated Seminal Vesicle Recurrence: 2 Case Reports. Appl Radiat Oncol. 2025;(1):34-36.
doi:10.37549/ARO-D-25-00001
March 1, 2025