RefleXion X1 Treatment Planning Feasibility Study for Craniospinal Irradiation (CSI)
Affiliations
- 1 The University of Texas MD Anderson Cancer Center, School of Health Professions, Houston TX
- 2 Department of Radiation Oncology, Stanford University, Stanford CA
- 3 Department of Radiation Oncology, University of Alabama at Birmingham, Birmingham AL
Objective:
The first clinical biology-guided radiation therapy system, RefleXion X1, was commissioned for clinical use at our institution. This study evaluates the X1 treatment planning feasibility of complex craniospinal targets for pediatric medulloblastoma patients and compares plan quality to multi-isocenter linac-based Volumetric Modulated Arc Therapy (VMAT) plans.
Methods:
Five pediatric patients treated with multi-isocenter VMAT craniospinal irradiation (CSI) were selected for this retrospective study. All planning target volumes (PTVs) had a craniocaudal length < 50 cm and received 36 Gy in 20 fractions. The target volumes and organs-at-risk (OARs) used for VMAT plans were utilized to generate plans using RefleXion X1. PTV D2%, OARs D mean and D max , and treatment times were collected for analysis. A paired-sample t -test was performed to detect significance at P < 0.05.
Results:
All 5 X1 CSI plans were successfully generated and deemed clinically acceptable for treatment. PTV D2% was found to be greater for X1 compared with VMAT plans ( P = .08). For the X1 plans, the D mean to the bowel, cochleas, heart, kidneys, lungs, and oral cavity was not found to be statistically significant ( P > .05) compared with VMAT plans. The average treatment beam-on time for X1 plans was 16.7 minutes vs 3.6 minutes for VMAT plans ( P < .01). However, the RefleXion X1 platform enables one isocenter treatment and 90-cm-long kilovoltage CT scan, which has the potential to reduce the setup/imaging time, and thus the total treatment time compared with multi-isocenter linac-based VMAT, where the total treatment time of up to 43.5 minutes was observed.
Conclusion:
Apart from a greater maximum dose to PTV, X1 plans showed comparable dosimetry to multi-isocenter VMAT plans. Although the average beam-on time with X1 was longer, there is a potential for a more streamlined setup and IGRT using a single isocenter plan.
Introduction
Medulloblastoma is the most common childhood malignant central nervous system tumor.1 Peak incidence occurs at age 7, with slightly greater incidence in males.1, 2 A large proportion of patients with medulloblastoma have craniospinal fluid spread at diagnosis. The standard of care for medulloblastomas involves surgical resection, craniospinal irradiation (CSI) with post fossa or surgical cavity bed boost to 54 Gy and chemotherapy.3 For average-risk patients, the 5-year survival rate is higher than 80%, while high-risk patients have a 5-year survival rate lower than 50%.2, 4
Craniospinal irradiation presents challenges because of its large target volume, which extends beyond the 40 cm × 40 cm field size limitation of a commonly used C-arm linear accelerator collimator opening.5, 6 The use of multiple plan isocenters overcomes this limitation by dividing the target volume into 3 fields: the whole brain, the upper spine, and the lower spine. Craniospinal irradiation is commonly performed using the 3D conformal radiation therapy technique, which is prone to errors owing to the complexity of the planning and the treatment delivery setup.7 - 12 This technique results in dose inhomogeneity and nonconformity, which yields significant dose to the anterior of the spine target volume. Three-dimensional CSI also requires feathering the junctions, resulting in multiple plan pairs, gap calculation, and couch rotations, making planning and treatment complex, cumbersome, and time consuming.
Overall, Volumetric Modulated Arc Therapy (VMAT) CSI creates plans with superior dose conformality, superior dose homogeneity, greater normal tissue sparing, lower sensitivity to positioning errors, and shorter treatment time compared with 3D CSI.13 - 17 While VMAT can produce clinically favorable plans even with setup errors of up to 3 mm, accurate patient alignment with minimal setup remains important. A multicenter study conducted by Gram et al11 showed that daily image guidance with 6-DoF couch corrections was found optimal in significantly reducing positioning errors and uncertainties for patients with pediatric CSI.
While daily image guidance and 6-degrees-of-freedom couch corrections can assist in optimizing patient setup, the inherent risks for positioning errors and uncertainties cannot be eliminated for VMAT CSI owing to the use of multiple isocenters and field matching. Helical-delivery radiation treatment techniques such as Tomotherapy can reduce these risks associated with multicenter CSI treatments by using a ring-based gantry to deliver a single-field CSI treatment as the patient moves into the treatment ring.18 - 20 A study by Lee et al19 reported Tomotherapy CSI to have acceptable inter- and intra-fractional errors, and setup verification based on the measurements and evaluations of treatment setup for 83 patients. In addition, Tomotherapy CSI techniques have been demonstrated to produce highly conformal and homogeneous treatment plans compared with 3D CSI.21 - 23
RefleXion (RefleXion Medical Inc) is a novel PET/CT treatment modality that similarly utilizes a ring-based gantry for axial step-and-shoot Intensity-Modulated Radiation Therapy (IMRT) delivery. The first clinical installation of RefleXion X1 was recently conducted at our institution.24, 25 The RefleXion X1 design provides potential advantages to CSI treatments using a single isocenter that can potentially decrease the complexity of planning, image guidance, and delivery, reducing the risk of shift and localization errors. This study aims to test the feasibility of treatment planning of X1 CSI and compare the plan quality and beam-on time to the current standard of care at our institution—VMAT CSI planned in Eclipse and delivered using Trilogy or TrueBeam linear accelerator (Varian Medical Systems).
Methods
Patient Selection and Simulation
Of 81 patients previously treated with VMAT CSI at Stanford University from 2012 to 2022, only 5 had a planning target volume (PTV) length of less than 50 cm in the craniocaudal direction (current RefleXion X1 TPS limitation). These 5 patients were included in this retrospective treatment planning feasibility study. Patients were simulated using a Siemens CT scanner (slice thickness 2 mm) in the head-first-supine position with arms by side, immobilized in a 5-point head and neck mask and AccuForm cushion (CIVCO) in the neutral neck position. All patients were treated under anesthesia.
VMAT CSI Planning
VMAT CSI plans were generated on Eclipse v15.6 (Varian Medical Systems) using 6 MV energy beams, a photon optimization algorithm, an analytical anisotropic algorithm dose calculation, and a calculation grid of 2.5 mm. Two full arcs were used to treat the brain and a single arc was used to treat the spine, with an overlap of at least 2 cm between the brain and spine fields. Brain and spine isocenters were placed such that there was only a longitudinal shift between them. Auto-feathering was enabled during optimization to create smooth dose gradients in the overlapping areas between fields. The spine arcs used avoidance sectors to limit the dose entering through the arms. VMAT CSI plans were normalized at 95% PTV coverage by the prescription dose of 36 Gy.
RefleXion X1 Linear Accelerator
RefleXion X1 is the first biology-guided radiation therapy system consisting of a 6 MV flattening-filter-free (FFF) linear accelerator mounted on the 85 cm gantry ring rotating at 60 rpm and delivering the treatment using one isocenter in axial fashion advancing the couch every 2.1 mm. Modulation is achieved using 64 binary, pneumatically driven, multi-leaf fast-transitioning collimators (MLC). Two sets of jaws, positioned above and below the MLCs, are used to set the maximum field extent in the patient superior-inferior direction: either 1 cm or 2 cm at isocenter. The X1 is also equipped with fan-beam kilovoltage CT of near-diagnostic image quality, megavoltage portal, and PET imaging subsystem.
RefleXion X1 Planning
CT scans and structure sets used for VMAT CSI plan generation were imported to RefleXion X1 TPS for planning. The PTV_CSI target ranged between 48.1 and 49.3 cm and was the same for VMAT and X1 planning. All cases were planned on the RefleXion X1 v1.0.46 TPS using step-and-shoot IMRT technique with 6 MV FFF energy, 2 cm jaws, accelerated proximal gradient-based on FISTA and Collapsed Cone Convolution superposition dose calculation algorithm, and a calculation grid of 2.1 mm. The plan isocenter was placed in the middle of the target. As plan dose normalization was not available in RefleXion X1 v1.0.46, each plan was optimized to allow for 95% of the PTV to receive the prescription dose (36 Gy in 20 fractions).
Plan Evaluation
Plans created in Eclipse and RefleXion X1 for each patient were evaluated for dose heterogeneity using dose to 2% of the PTV (D2%), conformity index, homogeneity index, and mean dose to critical structures.
Plan Comparison
A paired sample t -test was performed to evaluate the dosimetric quantities between the Eclipse and the RefleXion X1 plans for each patient, with statistical significance defined at P < 0.05.
Beam-on Time and Treatment Time Analysis
Beam-on times for Eclipse and RefleXion X1 TPS were collected and compared. The RefleXion X1 system dose rate used for the beam-on time study was 850 MU/min. Total time from imaging to end of treatment session was recorded using Aria offline review for Eclipse VMAT plans for every fifth fraction for each patient. Institutional guidelines for VMAT CSI treatment include imaging all isocenters separately using kV/kV orthogonal pairs and shifting and adjusting positioning to obtain an accurate match for each isocenter position. Cone beam CT is used for the first fraction and every fifth fraction or when alignment is problematic. After the imaging and adjustments, each isocenter position is confirmed with planar MV port added to the arc to confirm the accuracy of the shifts.
Results
RefleXion X1 plans were successfully created for all 5 patients with pediatric medulloblastoma. Figure 1 illustrates a comparison between axial and sagittal dose distributions between an Eclipse VMAT plan and a RefleXion X1 plan.
Comparison of sagittal (top) and axial (bottom) dose distributions between (A) Eclipse VMAT craniospinal irradiation (CSI) plan and (B) RefleXion X1 CSI plan. Colorwash dose threshold of 1800 cGy indicates 50% of prescription dose.
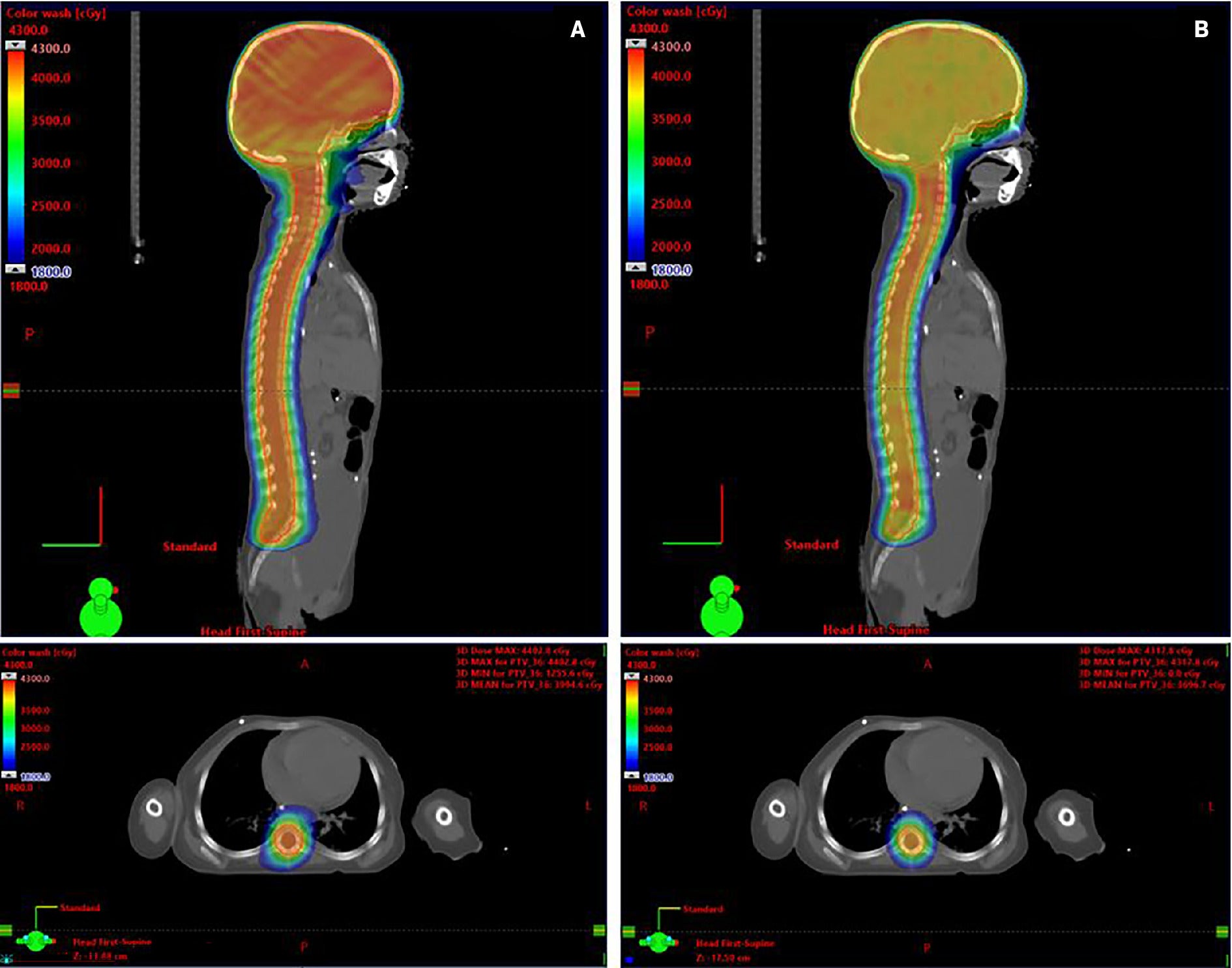
Table 1 displays the summary of the average dosimetric indices and parameters achieved for VMAT and X1 plans. The dose to 2% of PTV (PTV D2%) was reported as 39.2 Gy for VMAT plans and 41.3 Gy for X1 plans. This difference was not found to be statistically significant ( P = .08). The organs-at-risk (OAR) doses for the RefleXion X1 and Eclipse VMAT plans were comparable. However, all of the mean OAR doses were higher with the X1 even though the differences were not found to be statistically significant. Statistical significance was detected only for the difference in D mean to the bowel bag, with RefleXion X1 plans reporting a lower average D mean compared with Eclipse VMAT of 1.4 Gy ( P = .04).
Dosimetric and Time Parameters for RefleXion X1 and Eclipse VMAT Craniospinal Irradiation Plans
| RefleXion X1 | Eclipse VMAT | Difference | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structure | Parameter | Constraint | 1 | 2 | 3 | 4 | 5 | Average | SD | 1 | 2 | 3 | 4 | 5 | Average | SD | Difference | P value |
| PTV_CSI | D95% (Gy) | >36 | 36.3 | 35.9 | 35.6 | 36.1 | 36.9 | 36.2 | 0.5 | 36 | 36 | 36 | 36 | 36.1 | 36 | 0 | 0.2 | .53 |
| PTV_CSI | D2% (Gy) | <40.3 | 39.9 | 40.3 | 39.7 | 39.6 | 44.2 | 40.7 | 1.9 | 39.1 | 39.5 | 42.3 | 38.8 | 38.9 | 39.2 | 1.5 | 1.5 | .46 |
| PTV_CSI | CI | 1.00 | 1.01 | 0.99 | 0.89 | 0.96 | 1.12 | 0.99 | 0.1 | 0.97 | 0.97 | 1.05 | 0.93 | 0.96 | 0.98 | 0 | 0 | .74 |
| PTV_CSI | HI | 0 | 0.16 | 0.14 | 0.23 | 0.31 | 1.04 | 0.38 | 0.38 | 0.11 | 0.12 | 0.25 | 0.17 | 0.1 | 0.15 | 0.06 | 0.23 | .28 |
| BrainStem | D max (Gy) | <40.3 | 40.6 | 41.3 | 39.1 | 39.9 | 43.4 | 40.9 | 1.6 | 38.3 | 39.3 | 42.1 | 39.1 | 38.5 | 39.5 | 1.5 | 1.4 | .34 |
| BowelBag | D mean (Gy) | <10.0 | 7.5 | 11.4 | 7.4 | 12.1 | 13.5 | 10.4 | 2.8 | 8.3 | 13.4 | 9 | 12.1 | 16 | 11.7 | 3.2 | -1.4 | . 04 |
| Cochleas | D mean (Gy) | <39.6 | 38 | 37.7 | 37 | 38.3 | 41.7 | 38.5 | 1.8 | 37.9 | 38.4 | 40.2 | 37.4 | 38.1 | 38.4 | 1.1 | 0.1 | .93 |
| Esophagus | D mean (Gy) | <30.0 | 29.4 | 25.1 | 20.2 | 29.7 | 37 | 28.3 | 6.2 | 27.2 | 25.2 | 22.5 | 27.3 | 29.1 | 26.3 | 2.5 | 2 | .3 |
| Globes | D max (Gy) | <39.6 | 38.6 | 42.7 | 36.9 | 39.2 | 41.1 | 39.7 | 2.3 | 38.6 | 38.5 | 38.1 | 35 | 38.9 | 37.8 | 1.6 | 1.9 | .16 |
| Heart | D mean (Gy) | <12 | 11.5 | 12 | 7.6 | 13.6 | 17.6 | 12.5 | 3.6 | 10.8 | 10.6 | 9.9 | 12.9 | 13.8 | 11.6 | 1.7 | 0.9 | .43 |
| Kidneys | D mean (Gy) | <18 | 18.8 | 19.8 | 9.9 | 17.4 | 25.3 | 18.2 | 5.5 | 19.3 | 12.2 | 9.9 | 16.1 | 20.4 | 15.6 | 4.5 | 2.7 | .16 |
| Larynx | D mean (Gy) | <25 | 23.9 | 24.6 | 16.2 | 27.4 | 25.3 | 23.5 | 4.3 | 20.2 | 21.9 | 21.6 | 25.8 | 21.9 | 22.3 | 2.1 | 1.2 | .52 |
| Lungs | D mean (Gy) | <18 | 14 | 15.7 | 9 | 14.6 | 15 | 13.7 | 2.7 | 14 | 13.1 | 9.2 | 14.5 | 15.8 | 13.3 | 2.5 | 0.4 | .58 |
| OpticChiasm | D max (Gy) | <39.6 | 39.5 | 39.5 | 38.2 | 38.9 | 41 | 39.4 | 1 | 37.9 | 37.9 | 41 | 38.1 | 37.5 | 38.5 | 1.4 | 0.9 | .42 |
| OpticNerves | D max (Gy) | <39.6 | 38.2 | 40.6 | 37.8 | 39 | 40.4 | 39.2 | 1.3 | 38.6 | 38.4 | 40 | 38.1 | 38.5 | 38.7 | 0.7 | 0.5 | .57 |
| OralCavity | D mean (Gy) | <20 | 15.8 | 18.9 | 10.9 | 15.9 | 18.4 | 16 | 3.2 | 13.1 | 14.6 | 15 | 16 | 16.1 | 15 | 1.2 | 1 | .52 |
| Parotids | D mean (Gy) | <26 | 25.3 | 31.3 | 22.4 | 24.5 | 27.3 | 26.1 | 3.4 | 19.7 | 30 | 23.6 | 22.8 | 20.1 | 23.2 | 4.1 | 2.9 | .13 |
| Pituitary | D max (Gy) | <39.6 | 39.6 | 39.3 | 37.7 | 38.2 | 40.9 | 39.1 | 1.3 | 37 | 38.7 | 40 | 37.6 | 37.6 | 38.2 | 1.2 | 1 | .38 |
| SpinalCord | D max (Gy) | <40.3 | 42.5 | 41.6 | 42.2 | 40 | 45.4 | 42.3 | 2 | 40.4 | 41.9 | 42.3 | 39.7 | 40.4 | 40.9 | 1.1 | 1.4 | .23 |
| Submandibulars | D mean (Gy) | <20 | 19 | 20.9 | 13.7 | 20.6 | 20.4 | 18.9 | 3 | 17.8 | 18 | 19.1 | 20.3 | 17.9 | 18.6 | 1.1 | 0.3 | .85 |
| Thyroid | D mean (Gy) | <25 | 24.2 | 23.7 | 16.6 | 24.4 | 28.6 | 23.5 | 4.3 | 19.9 | 20.1 | 18.4 | 23.8 | 23.8 | 21.2 | 2.5 | 2.3 | .14 |
| Beam-on | Time (min) | 16.9 | 17.5 | 15.6 | 16.8 | 16.8 | 16.7 | 0.7 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 0 | 13.1 | <. 01 | |
| Total treatment | Time (min) | 43.5 | 22.3 | 41.6 | 16.3 | 22.4 | 29.2 | 12.4 |
Difference is an absolute difference between RefleXion X1 and Eclipse VMAT. Statistically significant P value <.05 is shown in bold font.
The average beam-on time for Eclipse VMAT and RefleXion X1 plans were 3.6 minutes and 16.7 minutes, respectively ( P < .01). The average total treatment time from imaging to completion of treatment for Eclipse VMAT was 29.2 minutes (range 16.3-43.5 min). No average total treatment time was acquired for RefleXion X1 because no treatment was delivered using this technology.
Discussion
To our knowledge, this is the first treatment planning study of CSI using the RefleXion X1 system. We have previously reported on treatment planning comparison between RefleXion X1 and Eclipse VMAT for 42 patients across 6 cancer sites.26 In this study, we tested the feasibility of CSI using RefleXion X1. We have successfully generated clinically acceptable RefleXion CSI plans for 5 pediatric medulloblastoma patients with target length less than 50 cm. Dosimetric indices were comparable between the RefleXion X1 and Eclipse VMAT modalities, except for statistically significantly improved bowel sparing with RefleXion X1.
Owing to the 2 cm field size and long PTV CSI targets, the average beam-on time was approximately 4.5 times greater using RefleXion X1 compared with Eclipse VMAT. For VMAT CSI delivery using 2-isocenter plans and implemented on a Varian C-arm linear accelerator, treatment times for the first fraction from start of imaging to completion of treatment ranged widely, from 16.3 to 43.5 minutes (mean, 29.2 min), signifying challenges in separately imaging and aligning each isocenter. RefleXion X1 can overcome this challenge by imaging a long extent of the patient, localizing, and delivering the whole treatment using one isocenter in axial mode and moving the couch in the craniocaudal direction with 2.1 mm increments. This may reduce beam matching and shifting errors that could arise from multi-isocenter delivery. In addition, X1 was recently upgraded to enable a 1000 MU/min dose rate from the initial dose rate of 850 MU/min improving the beam-on time.
While no studies currently compare RefleXion CSI and VMAT CSI, literature discussing the delivery of treatment using Tomotherapy with 2.5 cm jaws in helical fashion may be useful as a comparison due to its similarity to X1. A study in 2019 by Sun et al27 comparing VMAT, IMRT, and Tomotherapy plans found that the Tomotherapy plans offered superior PTV homogeneity, conformity, and brainstem, optic chiasm, and optic nerve sparing compared with those of VMAT plans. IMRT was superior to VMAT and Tomotherapy in terms of OAR sparing in the mid-body region (esophagus and heart). Results of this study by Sun et al differed from the results of the current RefleXion X1 study, which found difference in D mean to the bowel bag as the only statistically significant dosimetric parameter. However, just as the average beam-on time for RFX plans was estimated to be longer than the average beam-on time for VMAT plans in our study, Tomotherapy delivery time was found to be longer than that of VMAT by Sun et al. The long treatment time increases the potential for significant intrafraction motion. In future studies, the impact of intrafraction motion management on treatment time for RefleXion CSI will need to be evaluated.
Another study by Herdian et al28 found that differences in oral cavity D mean , kidneys D mean , and mean D2% to the spinal PTV were statistically significant between IMRT and Tomotherapy plans. Differences in oral cavity D mean , kidneys D mean , mean D2% to the cranial PTV, and mean D2% to the spinal PTV were also statistically significant between 3D-CRT plans and HT plans. Additionally, Tomotherapy plans resulted in longer mean beam-on times than both IMRT and 3D-CRT.28
One limitation of this study is the small sample size ( n = 5) due to the maximum target length threshold of 50 cm. The vendor is planning in its next clinical release to upgrade the system with the capability to treat targets greater than 50 cm. This will permit us to expand patient selection, include larger target sizes, and collect and further analyze treatment delivery times. In the system’s current version, treatment would require an additional plan to cover the entire target. Future studies will have to explore the issue of field matching in these situations. Another limitation is that this study focuses only on comparing the VMAT and RefleXion X1 plans. It would be interesting to include Tomotherapy plans in the testing cohort. This work shows the feasibility of CSI planning using RefleXion X1, potentially paving the way to use RefleXion X1 for CSI treatment. This could simplify Image-Guided Radiation Therapy (IGRT) workflow and streamline treatment, an especially important benefit for patients with pediatric CSI being treated under anesthesia.
Conclusion
Based on our limited data set, we were able to demonstrate the feasibility of CSI treatment planning for RefleXion X1. The successfully generated RefleXion plans resulted in dosimetric indices comparable to Eclipse VMAT plans as no statistically significant differences were detected in the PTV near-maximum dose or average D mean to critical structures except in the bowel bag. Despite its longer average beam-on time than VMAT plans, RefleXion X1 utilizes a moving couch to allow for single-isocenter technique by encompassing the entire volume in one scan. This has the potential to reduce translational and dosimetric matching errors associated with multi-isocenter setups using C-arm linear accelerators.
References
Citation
T N, P P, IO R, E S, M HBS, I G, S H, N K. RefleXion X1 Treatment Planning Feasibility Study for Craniospinal Irradiation (CSI). Appl Radiat Oncol. 2025;(1):27-33.
doi:10.37549/ARO-D-24-00032
March 1, 2025