Investigational Embolic Device Selected for “Best of GEST” at Symposium
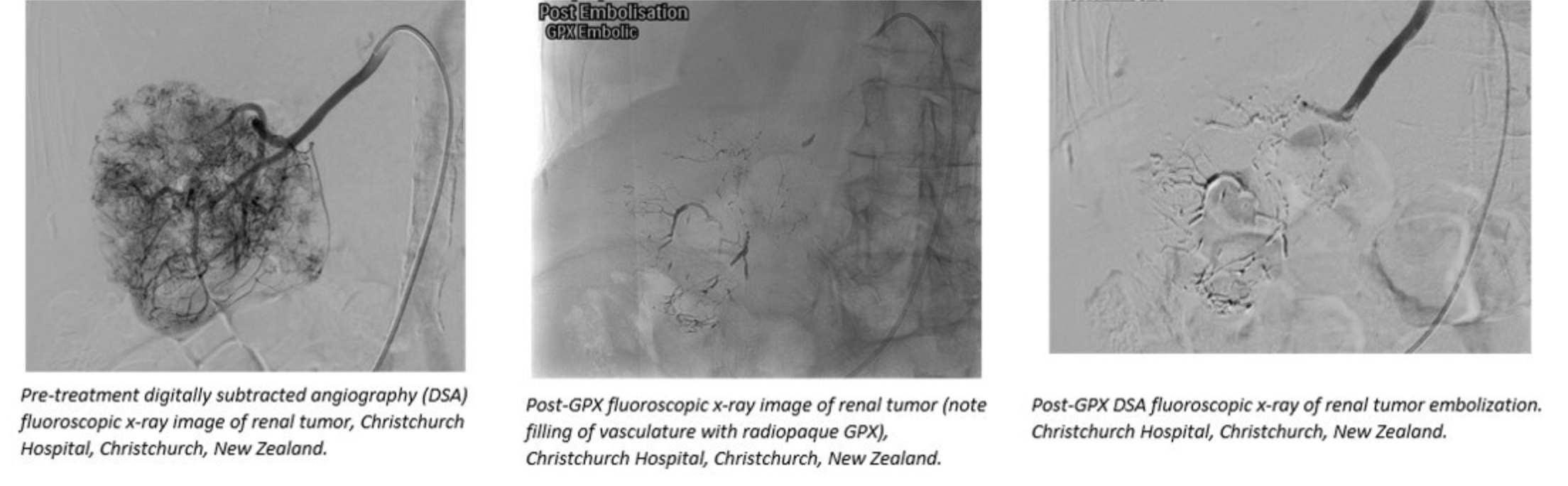 The GPX Embolic Device from Fluidx Medical Technology was selected for a Global Embolization Oncology Symposium Technologies ("GEST") "BEST of GEST" session highlighting the technology's ability to devascularize tumors during minimally-invasive transcatheter embolization procedures. The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time, and is for investigational use in New Zealand only.
The GPX Embolic Device from Fluidx Medical Technology was selected for a Global Embolization Oncology Symposium Technologies ("GEST") "BEST of GEST" session highlighting the technology's ability to devascularize tumors during minimally-invasive transcatheter embolization procedures. The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time, and is for investigational use in New Zealand only.
The GPX Embolic Device is an innovative embolic designed for simple preparation and quick material delivery, as highlighted in case videos at the BEST of GEST event. GPX technology is a low viscosity, aqueous-based solution in a syringe that solidifies into a durable embolus upon delivery without polymerization or dimethyl-sulfoxide (DMSO) precipitation. GPX is designed to occlude blood vessels independent of a patient's coagulation situation.
"The versatility of the GPX product has been demonstrated in a variety of interventional oncology uses," said Andrew Holden, M.D., MBChB, FRANZCR, EBIR, ONZM, Director of Interventional Radiology, Auckland City Hospital, Auckland, New Zealand. "We have seen excellent distal penetration and backfilling of larger vessels in our tumor cases. In these cases, GPX provided effective, durable occlusions as confirmed using angiography several weeks post-GPX delivery."
According to the company, GPX is packaged ready-to-use in a syringe, requires less than 1 minute of tableside preparation by the clinician, and may be delivered through standard catheters or small microcatheters; no complex mixing systems or special delivery catheters are necessary.