AI-Powered Prostate Cancer Precision Oncology Platform Receives FDA Clearance
 Avenda Health announced the US FDA has cleared its cancer management platform, iQuest, designed to provide personalized care for prostate cancer patients.
Avenda Health announced the US FDA has cleared its cancer management platform, iQuest, designed to provide personalized care for prostate cancer patients.
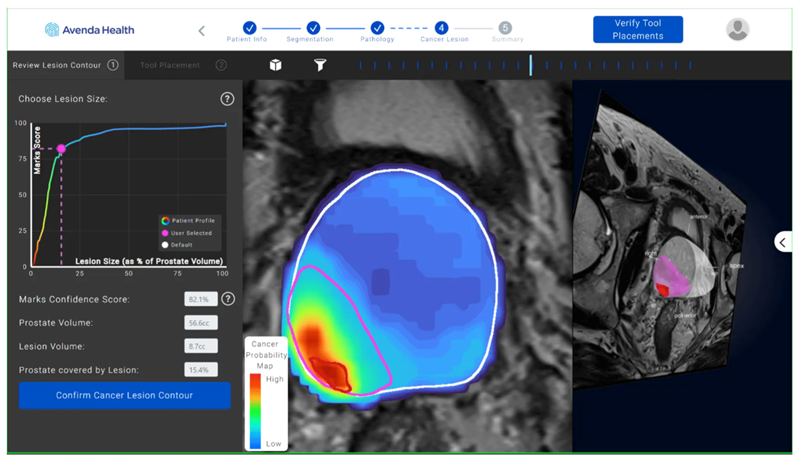
iQuest combines existing patient-specific diagnostic information and deep-learning algorithms to create a tailored map of where cancer is within the prostate that for the first time will allow for more personalized, effective and precise treatments. The platform enables a physician to make decisions that consider the cancer extent rather than treating the whole organ. This information supports physicians and patients with treatment selection, planning, guidance, and follow-up.
"We are excited about the potential to unlock precision care in prostate cancer with iQuest, as it is a key enabling technology for focal therapy to be a reality for urologists and patients," said Dr Shyam Natarajan, co-founder and CEO of Avenda Health. "In order for a doctor to treat focally, they need to know where cancer is and the healthy tissue to avoid. This is vital information that iQuest now provides. This is a huge step forward in transforming the standard of care in prostate cancer and brings us that much closer to offering effective therapy that preserves quality of life to providers and patients across the US."
One in eight men will develop prostate cancer in their lifetime. The current one-size-fits-all approach of treating the entire prostate‒because current MRI technology cannot identify the full extent of the tumor and cancer growth within the prostate‒results in nearly 50 percent of patients losing their sexual or urinary function. iQuest personalizes treatment options for the individual patient in order to preserve quality of life and prevent recurrence. Providing physicians with a 3D visualization of the cancer offers a better understanding of the extent of the disease to aid in treatment planning that can preserve quality of life while minimizing cancer left behind.
iQuest was built upon a decade of research from hundreds of thousands of data points and validated in multiple clinical studies. In one study, urologists improved their sensitivity of identifying tumor extent by using iQuest from 37% to 97% and changed their treatment recommendation in 27% of cases, with the biggest change towards a more localized treatment. iQuest can be applied to multiple treatment options to guide a doctor's intervention plan, including FocalPoint—Avenda Health's soft tissue laser ablation device—and other focal treatments, active surveillance, and decision-making for whole gland treatments including radical prostatectomy and radiation therapy.
"At the heart of what we do is the belief that patients should never have to choose between quality of life and cancer control," said Dr Brittany Berry-Pusey, co-founder and COO of Avenda Health. "By providing a personalized cancer map, iQuest opens the door for more precise treatments. This clearance will go a long way not only for our company but for the future of prostate cancer care."