Total body irradiation: A practical review
Images


Total body irradiation (TBI) with megavoltage photon beams is one component used in treating several diseases, including multiple myeloma, leukemias, lymphomas and some solid tumors.1,2 In combination with chemotherapy, TBI is most commonly used as part of the conditioning regimen prior to hematopoietic stem cell transplantation.1,3,4 TBI provides a uniform dose of radiation to the entire body, penetrating areas such as the central nervous system (CNS) and testes, where traditional chemotherapy is ineffective.5,6 Additionally, it allows tailoring of therapy with the ability to shield or boost the dose to certain regions as necessary. The purpose of TBI is threefold: to eliminate residual cancer cells, to provide space for stem cell engraftment through bone marrow depletion, and to prevent rejection of donor stem cells through immunosuppression.3,4
Dosing
The reported D0 value—the amount of ionizing radiation necessary to eradicate a particular cell type—of hematopoietic stem cells is 0.5 to 1.4 Gy, while those of human leukemia cell lines are 0.8 to 1.5 Gy, indicating that both cells are radiosensitive.4 The ideal dosing schedule depends on patient age, disease and the intended type of stem cell transplant.6 Recommendations state that the most common dose schedule for myeloablative TBI is 12 to 15 Gy given in 8 to 12 fractions over 4 days, with 2 to 3 treatments daily.6-8 Doses > 15 Gy have been shown to decrease relapse rate, but also increase the incidence of graft vs. host disease and decrease 2-year survival.7-9 Dose rates are often 6 to 15 cGy/min, consistent with recommendations of the American Association of Physicists in Medicine (AAPM) TG-17 report, as it has been reported that dose rates < 20 cGy/min help reduce complications.10 Low-dose TBI, with doses of 2 to 8 Gy given in 1 to 4 fractions in combination with chemotherapy, is an effective conditioning regimen for hematopoietic stem cell transplantation in patients who cannot tolerate myeloablation due to age or comorbidities.6,11 Fractionated TBI has been shown to lead to a higher incidence of graft rejection than the same dose delivered in a single fraction, possibly due to DNA repair during interfraction intervals.4,7,12 However, fractionation decreases the eradication of bone marrow stromal cells, which are necessary for successful hematopoietic stem cell engraftment, and is, therefore, considered the standard of treatment.4,6 Whole-dose inhomogeneity should be maintained within ± 10% to minimize the risk of complications.6 The AAPM TG-29 report provides instructions for dose prescription calculations.13 To perform these calculations, patient thickness should be measured at the prescription point, generally the level of the umbilicus.6 One method to independently verify the accuracy of delivery is to perform in-vivo measurements. Penn State uses Landauer (Glenwood, Illinois) nanoDot OSLD dosimeters at the umbilicus position for the AP field, and an umbilicus-equivalent position facing the beam for the PA field, with ± 5% tolerance as advised.14
Equipment
Guidelines recommend the use of parallel opposed pairs of high-energy photon beams from 4 to 18 MV for TBI;1,6 in our institutions we use 6 MV to avoid underdosing superficial bones such as the iliac crest and sternum. AAPM’s TG-51 calibration protocol provides guidelines for dosimetry of high-energy photon beams.15 Recent studies demonstrate the efficacy of helical tomotherapy and dynamic arc-based techniques for decreasing TBI treatment time and increasing homogeneity of delivered radiation; however, the use of this technique is not widespread.16-19
At Penn State, a Varian Clinac iX is used for TBI, and at Cleveland Clinic, a Siemens Artiste is used. In both institutions, another linear accelerator is identified as a backup in case the primary treatment machine goes down. At Cleveland Clinic, this is an identical Siemens Artiste, and at Penn State, it is a Varian Trilogy. At Penn State, both linear accelerators were commissioned using the same source-to-surface distance (SSD = 463 cm). The absolute dose for both machines was calibrated at 100 cm SAD (surface to axis distance) using a 10-x-10-cm field size according to the AAPM TG-51 protocol, but TBI treatments are delivered using a larger field (40-x-40-cm) and extended SSD. Thus, the dosimetry tasks for TBI commissioning included: a) measuring the output factor at the central point of treatment distance; b) generating the table of tissue maximum ratio (TMR) at the central point of treatment distance; and c) measuring the screen factor. At Penn State, this was performed using a PTW TN30013 ion chamber (PTW, Freiburg, Germany), a Fluke electrometer (Fluke Biomedical, Everett, Washington), and multiple 30-x-30-cm PVC phantoms. To independently verify dosimetrical accuracy, in-vivo measurements with nanoDot OSLD dosimeters14 were performed with PVC phantoms after commissioning.
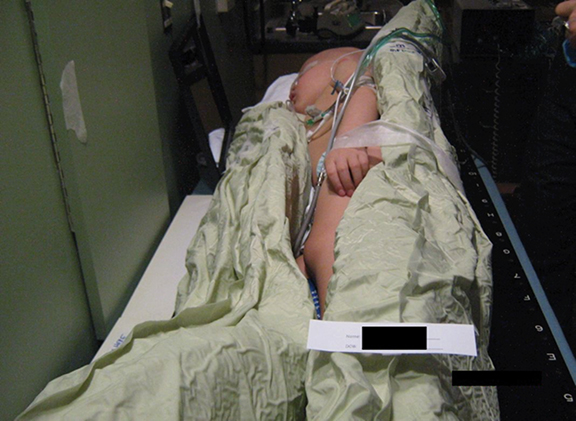
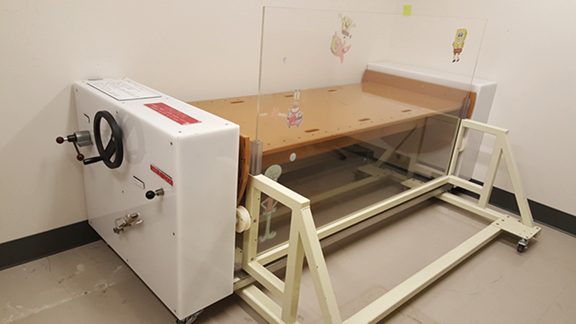
When opposing photon beams are used for TBI, patients are treated with 2 parallel-opposed fields, with each field treated in each fraction. If a single source of radiation is used, the patient is rotated 180 degrees along the longitudinal axis between doses.4 For each field, the coronal midline of the patient is aligned with the treatment plane marked on the floor at the time of commissioning. Irradiation along the anterior-posterior/posterior-anterior (AP/PA) direction provides better dose uniformity.4 TBI stands, treatment couches or tables are used to immobilize the patient lying supine/prone or standing upright if a vertical beam is used, or with the patient on his or her side if a horizontal beam is used. Pediatric patients under anesthesia may need to be irradiated using a lateral beam while lying supine due to airway concerns, but this technique should be avoided when possible for patients with large lateral separations.4,6 Different setups and equipment used at the Penn State Cancer Institute are shown in Figures 1-3. Unlike conventional radiation therapy in which skin sparing is often desired, it is preferable for the skin to receive a full dose of radiation for certain types of diseases treated with TBI, such as leukemias that can circulate in the blood volume of the skin.4 Beam spoilers scatter electrons as photons from the TBI beam pass through them, allowing energy to deposit near the surface of the skin.4,20
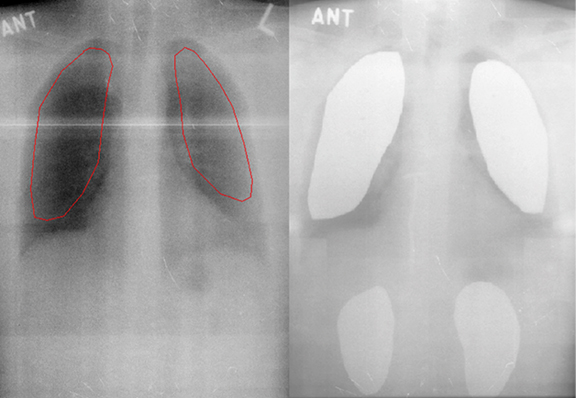
Lung shielding using lead or alloy attenuators, which reduce radiation dose to the majority of lung tissue, is recommended during normal—but not low—dose TBI to reduce the risk of pneumonitis, particularly in patients with concomitant lung dysfunction.4,6,21 However, overcompensation through the use of lung shields can increase the risk of leukemia recurrence, so shields should generally correspond to a 10% to 50% reduction in radiation dose.4 Lung thickness, size and density must all be considered when calculating radiation dose to the lungs.1 Lung shields can be tailored to avoid shielding the thymus, hilum, thoracic vertebrae, and heart. An example of a radiograph showing lung shield placement and its corresponding digitally reconstructed radiograph produced during the planning process is shown in Figure 4.
Literature demonstrating the benefit of lung blocking is limited, with only small retrospective series available. One such study assessed 44 patients receiving 12 Gy TBI in 6 fractions over 3 days.22 Twenty-three patients received this regimen without shielding and the remaining 21 received lead shielding to 50% dose reduction after the first 6 Gy, yielding a total lung dose of 9 Gy. Over the next 6 months, 6 out of the 23 patients (26%) who did not receive shielding developed interstitial pneumonitis, diagnosed either clinically with cough, dyspnea, or radiographically as bilateral interstitial infiltrates without an infectious etiology.22 In half of these cases, the complication was fatal. No one who received shielding developed interstitial pneumonitis.22 Although this level of evidence is not definitive, given the potential of lethality if interstitial pneumonitis develops, the Children’s Oncology Group recommended, but did not require, the use of lung blocks in recent protocols, such as ASCT (autologous stem cell transplant) 0631.
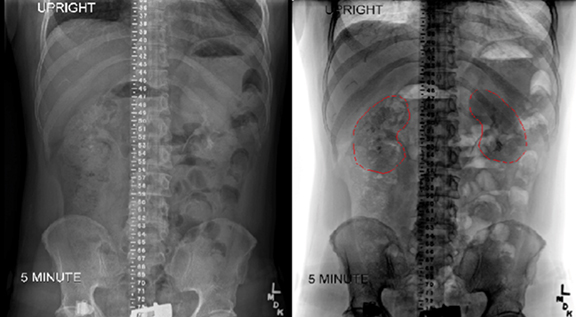
Renal shielding is another common technique for reducing the level of radiation delivered to the kidneys. Bone marrow transplant nephropathy, consisting of renal dysfunction with hypertension, proteinuria, edema, anemia, and decreased glomerular filtration rate, is a serious possible complication of TBI.23 Because kidneys are not a sanctuary site, kidney blocks have been used standardly at the Cleveland Clinic. The kidneys shift inferiorly significantly when patients move from a supine to upright position, so kidney blocks should be designed based on scans performed in the desired TBI position.24,25 At the Cleveland Clinic, an intravenous urogram in the standing position is performed at 5, 10, and 15 minutes, and the image with the best kidney outline is used for block design. Additionally, the radiologist reports exactly at what distance the top and bottom of the kidneys are with reference to the central distance marker. Examples of radiographs showing the placement of kidney and the combination of lung and kidney shields from the Cleveland Clinic are shown in Figure 4.
As with lung blocks, the level of evidence in support of kidney blocks is limited to retrospective studies. One such example assessed 157 patients receiving 14 Gy TBI and surviving at least 100 days for the development of nephropathy over 2.5 years from treatment. The authors report a nephropathy rate in the 72 patients who did not receive kidney shielding of 29 +/- 7% and 14+/- 5% in the 68 patients who received 15% renal shielding. No incidents were reported in the 17 patients who received 30% kidney shielding. The authors concluded that shielding should be used in those who require doses > 12 Gy.23 Although this is standard practice at the Cleveland Clinic, it is not the practice at the Penn State Cancer Institute, which follows Children’s Oncology Group protocols whereby only lung shields, and not kidney shields, are allowed. Finally, both gonad and thymus shielding have been used by some clinicians,21 but are not used in either of our institutions.
Complications
Without careful medical monitoring and hematopoietic stem cell transplantation, TBI is a potentially fatal therapy.6 Immediately following TBI, the most common acute symptoms include nausea, emesis, loss of appetite, diarrhea, mild erythema, pruritus, headache, xerostomia, parotitis and fatigue syndrome.26 Therapies to control these side effects include intravenous hydration, antimucositis and antiemetic agents.6,27 Long-term complications of TBI include secondary malignancies, infertility, cardiovascular disease, pneumonitis, nephritis, cataracts, and learning deficits and growth failure in children.6,28 Attention to calculations and careful technique is critical to minimize the risk of late-term sequelae.
Indications
TBI is used as part of the conditioning regimen for both autologous and allogeneic hematopoietic stem cell transplantations. A study of German stem cell transplant patients by Heinzelmann et al found that approximately 10% of autologous transplant patients receive TBI, with chronic lymphocytic leukemia (80%) and non-Hodgkin’s lymphoma (35%) being the most common disorders for which TBI was used.29 The same study found that 50% of allogeneic transplant patients received TBI, with acute lymphocytic leukemia (85%), acute myeloid leukemia (45%) and chronic myeloid leukemia (49%) being the most common disorders.29
Acute Lymphoblastic Leukemia
Acute lymphoblastic leukemia (ALL) is a disorder of malignant lymphoid progenitor cells. Although ALL affects children and adults, the majority of patients are diagnosed between ages 2 to 5 years.30 Approximately 6,000 cases of ALL are diagnosed annually in the United States, many of which are idiopathic. The majority of initial treatment regimens for ALL, which include a remission-induction phase, an intensification phase and continuation therapy, achieve overall disease-free survival rates of 80% to 90%.30 Both allogeneic and autologous stem cell transplantation have been successfully used in treating ALL, but allogeneic transplants are more common.31 Transplantation is the most intensive type of therapy for ALL and is typically considered in patients with high-risk ALL (such as those with Philadelphia chromosome-positive disease), those with early relapse (within 3 years of primary remission), or those who have a poor response to induction therapy.3,30 Long-term survival rates > 65% have been demonstrated for ALL patients transplanted during the first relapse.32,33 In a retrospective study using data from the International Bone Marrow Transplant Registry by Davies et al that compared cyclophosphamide plus TBI (CY/TBI) vs. busulfan plus cyclophosphamide (Bu/CY) conditioning regimens for childhood ALL, CY/TBI was found to have a higher 3-year leukemia-free survival rate (55% vs. 40%), lower treatment-related mortality, and a lower rate of treatment failure compared to Bu/CY.34 The addition of etoposide to the CY/TBI regimen may also improve survival.35
Acute Myeloid Leukemia
Acute myeloid leukemia (AML) is a disorder of the myeloid cell lineage, characterized by rapid growth and arrested maturation of cells.36 AML is the most common acute adult leukemia, with an incidence of approximately 2.4/100,000 in the United States. Despite improvements in treatment, the survival rate of patients under age 65 is < 50%.3,36 Treatment for AML is typically divided into induction and postinduction phases. Options for postinduction therapy consist of allogeneic bone marrow transplantation, autologous transplantation, or chemotherapy. Allogeneic transplants can cure 50% to 60% of recipients and have relapse rates of < 20%,36-38 while autologous transplants have survival rates of 45% to 55%.41 Greater leukemia control can be obtained through the use of conditioning regimens with TBI (such as CY/TBI), but the survival rate is comparable to chemotherapy combinations.40
Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL) is a malignancy of disordered apoptosis and proliferation of the lymphoid cell lineage. It is the most common type of leukemia in North America and Europe, and predominantly affects adults.41 Unlike ALL and AML, CLL is incurable and, although treatment exists, most patients relapse. There are generally 3 subsets of CLL patients: one-third experience slow disease progression with treatment consisting of watchful waiting, one-third exhibit an indolent phase followed by progression, and one-third need direct treatment for aggressive disease.41 Chemotherapy or autologous stem cell transplants are used to aid remission efforts. However, Ritgen et al found that an unmutated variable heavy-chain gene plays a role in whether the transplant is successful.42 Relapse was inevitable in the group with unmutated genes, whereas patients with mutated heavy-chain genes went into remission following autologous transplant.42 In elderly patients, the myeloablative conditioning regimen for transplant has a treatment-related mortality of 40% to 50%, so lower doses of radiation are typically used.43
Chronic Myeloid Leukemia
Chronic myeloid leukemia (CML) is a disorder of malignant myeloid cells. It was the first leukemia for which a distinct chromosomal aberration, the 9;22 translocation that results in a BCR-ABL fusion gene, or Philadelphia chromosome, was discovered.44 CML is relatively rare, with an incidence of 1 to 2 per 100,000 people, and is more common in the elderly population.44 Imatinib, a drug that competitively binds to and inhibits the BCR-ABL tyrosine kinase, is the treatment standard and results in up to an 87% remission.44,46 Allogeneic stem cell transplants are recommended as second-line therapy if imatinib fails, or in cases of high-risk disease.46 Five-year survival rates after allogeneic transplants are around 50%, with relapse rates around 20%.46 Reduced-dose TBI has been effective in lowering morbidity associated with myeloablation, but is not standard.43
Multiple Myeloma
Multiple myeloma is a malignant monoclonal proliferation of plasma cells, and accounts for 13% of hematologic cancers.47 Interactions between malignant plasma cells and bone marrow cells increase tumor growth and progression.47 Treatment for multiple myeloma depends on disease severity and patient age. Active or symptomatic disease requires immediate treatment, whereas asymptomatic disease only necessitates clinical observation.47 Symptomatic patients under age 65 who present without significant co-morbidities should be started on chemotherapy plus stem cell transplantation. Patients over age 65 or those with co-morbidities should be evaluated for autologous stem cell transplantation with low-intensity conditioning, or remain on traditional chemotherapy regimens.47
Lymphoma
There are many types of lymphoma, which can be divided into the categories of Hodgkin lymphoma, which is characterized by the presence of Reed-Sternberg cells, and non-Hodgkin lymphoma, which encompasses all other types of lymphoma. Treatment of Hodgkin lymphoma typically includes chemotherapy followed by involved-field radiotherapy or involved-site radiotherapy, which target specific lymph nodes rather than the entire body.48 Stem cell transplants are used as second line therapy for Hodgkin lymphoma that is difficult to treat or unresponsive to traditional therapy. Chemotherapy is the standard treatment for non-Hodgkin lymphoma, and stem cell transplants are only considered for patients unresponsive to chemotherapy, although new protocols are under investigation.49,50
Melanoma
Melanoma is a type of skin cancer with a lifetime risk of 1 in 59 in the United States.51 The most common risk factor for melanoma is sun exposure, and surgical removal is the treatment standard for cutaneous melanoma with negative lymph nodes.52 Metastatic melanoma is treated with chemotherapy or immunotherapy, including interleukin-2 or interferon alpha.52 Adoptive cell transfer therapy is a relatively new treatment option that has shown antitumor responses in > 50% of patients with advanced-stage melanoma. In this treatment, chemotherapy is used to reduce host lymphocytes, and T cells harvested from tumors or peripheral blood that are specific for cancer antigens are infused into a patient.52,53 TBI can be used as part of the conditioning regimen before adoptive cell transfer, and is associated with higher tumor response.53,54
Conclusion
TBI is an effective component of conditioning for hematopoietic stem cell transplant procedures. Although several adverse side effects are associated with TBI, treating various forms of leukemia and lymphoma with transplantation remains one of the most successful forms of therapy. More research is needed on the effects of low dose or nonmyeloablative irradiation, particularly for elderly patients, to reduce treatment-related morbidity and mortality. In addition, research on faster, more uniform methods of radiation delivery, such as helical tomotherapy, may make TBI more accessible to a wider spectrum of patients. For centers interested in starting a TBI program, we recommend following appropriate AAPM reports referenced above; having an identified, commissioned backup treatment machine in case of primary machine downtime; and following cooperative group or IRB-approved research protocols for treatment delivery.
References
- Levitt, SH, Purdy JA, Perez CA, eds. Technical Basis of Radiation Therapy: Practical Clinical Applications. Berlin, Germany: Springer; 2006.
- Zheng Y, Dou Y, Duan L, et al. Using chemo-drugs or irradiation to break immune tolerance and facilitate immunotherapy in solid cancer. Cellular Immunology. 2015;294(1):54-59.
- Halperin E, Constine L, Tarbell N, Kun L. Pediatric Radiation Oncology 4th Edition. Philadelphia: Lippincott Williams & Wilkins; 2005.
- Halperin E, Wazer D, Perez C, Brader L. Perez and Brady’s Principles and Practice of Radiation Oncology 6th Edition. Philadelphia: Lippincott Williams & Wilkins; 2013.
- Khan F. The Physics of Radiation Therapy 3rd Edition. Philadelphia: Lippincott Williams & Wilkins; 2003.
- Seung S, Larson D, Galvin J, et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for the Performance of Stereotactic Radiosurgery (SRS). Am J Clin Oncol. 2013;36(3):310-315.
- Storb R, Raff RF, Appelbaum FR, et al. Fractionated versus single-dose total body irradiation at low and high dose rates to condition canine littermates for DLA-identical marrow grafts. Blood. 1994;83(11):3384-3389.
- Storb R, Raff RF, Appelbaum FR, et al. Comparison of fractionated to single-dose total body irradiation in conditioning canine littermates for DLA-identical marrow grafts. Blood. 1989;74:1139-1143.
- Baron F. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23(9):1993-2003.
- Buchali A, Feyer P, Groll J, et al. Immediate toxicity during fractionated total body irradiation as conditioning for bone marrow transplantation. Radiother Oncol. 2000;54(2):157-162.
- Hongeng S, Krance RA, Bowman LC, et al. Outcomes of transplantation with matched-sibling and unrelated donor bone marrow in children with leukaemia. Lancet. 1997;350(9080):767-771.
- Alyea E, Neuberg D, Mauch P, et al. Effect of total body irradiation dose escalation on outcome following t-cell-depleted allogeneic bone marrow transplantation. Biol Blood and Marrow Transplant. 2002;8(3):139-144.
- Van Dyk J, Galvin JM, Glasgow GP, Podgorsak EB. The physical aspects of total and half body photon irradiation. AAPM Report No. 17. 1986. https://www.aapm.org/pubs/reports/RPT_17.pdf.
- Kim DW, Chung WK, Shin DO, et al. Dose response of commercially available optically stimulated luminescent detector, AI203:C for megavoltage photons and electrons. Radiat Prot Dosimetry. 2012;149(2):101-108.
- McEwen M, Dewerd L, Ibbott G, et al. Addendum to the AAPM’s TG-51 Protocol for Clinical Reference Dosimetry of High-energy Photon Beams. Med Phys. 2014;41(4):041501.
- Hui SK, Kapatoes J, Fowler J, et al. Feasibility study of helical tomotherapy for total body or total marrow irradiation. Med Phys. 2005;32(10):3214-3224.
- Gruen A, Ebell W, Wlodarczyk W, et al. Total body irradiation (tbi) using helical tomotherapy in children and young adults undergoing stem cell transplantation. Radiat Oncol. 2013;8(1):92.
- Takahashi Y, Vagge S, Agostinelli S, et al. Multi-institutional feasibility study of a fast patient localization method in total marrow irradiation with helical tomotherapy: a global health initiative by the International Consortium of Total Marrow Irradiation. Int J Radiat Oncol Biol Phys. 2015; 91(1):30-38.
- Jin JY, Wen N, Ren L et al. Advances in treatment techniques. Cancer J. 2011;17(3):166-176.
- Ravichandran R, Binukumar JP, Davis CA, et al. Beam configuration and physical parameters of clinical high energy photon beam for total body irradiation (TBI). Phys Med. 2011;27(3):163-168.
- Labar B, Nemet D, Bogdanic V, et al. Total body irradiation with or without lung shielding for allogeneic bone marrow transplantation. Bone Marrow Transplant. 1992;9(5):343-347.
- Weshler Z, Breuer R, Or R, et al. Interstitial pneumonitis after total body irradiation: effect of partial lung shielding. Br J Haematol. 1990;74(1):61-64.
- Lawton CA, Cohen EP, Murray KJ. Long-term results of selective renal shielding in patients undergoing total body irradiation in preparation for bone marrow transplantation. Bone Marrow Transplant. 1997;20(12 ):1069-1074.
- Reiff J, Werner-Wasik M, Valicenti R, Huq S. Changes in the size and location of kidneys from the supine to standing and the implications for black placement during total body irradiation. Int J Radiat Oncol Biol Phys. 1999;45(2)447-449.
- Cracinescu O, Steffey B, Kelsey C, et al. Renal shielding and dosimetry for patients with severe systemic sclerosis receiving immunoablation with total body irradiation on the SCOT scleroderma: cyclophosphamide or transplantation trial. Int J Radiat Oncol Biol Phys. 2011;79(4):1248-1255.
- Leiper AD. Late effects of total body irradiation. Arch Dis Child. 1995;72(5):382-385.
- Matsuoka S, Okamoto S, Watanabe R, et al. Granisetron plus dexamethasone versus granisetron alone in the prevention of vomiting induced by conditioning for stem cell transplantation: a prospective randomized study. Int J Hematol. 2003;77(1):86-90.
- Ozsahin M, Belkacemi Y, Pens F, et al. Total-body irradiation and cataract incidence: a randomized comparison of two instantaneous dose Rates. Int J Radiat Oncol Biol Phys. 28.2 (1994):343-347.
- Heinzelmann F, Ottinger H, Müller C-H, et al. Total-body irradiation—role and indications. Strahlenther Onkol. 2006;182(4):222-230.
- Pui C-H, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030-1043.
- Marks DI, Forman SJ, Blume KG, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12(4):438-453.
- 3Woolfrey AE. Factors associated with outcome after unrelated marrow transplantation for treatment of acute lymphoblastic leukemia in children. Blood. 2002;99(6):2002-2008.
- Appelbaum FR. Bone marrow transplantation or chemotherapy after remission induction for adults with acute nonlymphoblastic leukemia. Ann Intern Med. 1984;101(5):581.
- Davies SM, Ramsay NK, Klein JP, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000;18(2):340-347.
- Biagi E, Rovelli A, Balduzzi A, et al. TBI, etoposide and cyclophosphamide as a promising conditioning regimen for BMT in childhood all in second remission. Bone Marrow Transplant. 2000;26(11):1260-1262.
- Löwenberg B, Downing J, Burnett A. Acute myeloid leukemia. New Engl J Med. 1999;341:1051-1062.
- Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867-1871.
- Duerst RE, Horan JT, Liesveld JL, et al. Allogeneic bone marrow transplantation for children with acute leukemia: cytoreduction with fractionated total body irradiation, high-dose etoposide and cyclophosphamide. Bone Marrow Transplant. 2000; 25(5):489-494.
- Löwenberg B, Abels J, Van Bekkum DW, et al. Transplantation of non-purified autologous bone marrow in patients with AML in first remission. Cancer. 1984;54(12):2840-2843.
- Jung AS, Holman PR, Castro JE, et al. Autologous hematopoietic stem cell transplantation as an intensive consolidation therapy for adult patients in remission from acute myelogenous leukemia. Biol Blood Marrow Transplant. 2009;15(10):1306-1313.
- Le Dieu R, Gribben JG. Transplantation in chronic lymphocytic leukemia. Curr Hematol Malig Rep. 2007;2(1):56-63.
- Ritgen M, Lange A, Stilgenbauer SR, et al. Unmutated immunoglobulin variable heavy-chain gene status remains an adverse prognostic factor after autologous stem cell transplantation for chronic lymphocytic leukemia. Blood. 2002;101(5):2049-2053.
- Gratwhol A, Brand R, Apperley J. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the chronic leukemia working party of the European Group for Blood and Marrow Transplantation. Haematologica. 2006;91(4):513-521.
- Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370(9584):342-350.
- O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994-1004.
- Or R. Nonmyeloablative allogeneic stem cell transplantation for the treatment of chronic myeloid leukemia in first chronic phase. Blood. 2002;101(2): 441-445.
- Palumbo A, Anderson K. Multiple myeloma. New Engl J Med. 2011;364(11):1046-1060.
- Kuppers R, Engert A, Hansmann M-L. Hodgkin lymphoma. J Clin Invest. 2012;122(10):3439-3447.
- Minard-Colin V, Brugieres L, Reiter A, et al. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33(27):2963-2974.
- Isidori A, Clissa C, Loscocco F, et al. Advancement in high dose therapy and autologous stem cell rescue in lymphoma. World J Stem Cells. 2015;7(7): 1013-1046.
- Rigel, DS. Epidemiology of melanoma. Sem Cutan Med Surg. 2010;29(4):204-209.
- Schadendorf D, Fisher D, Garbe C, et al. Melanoma. Nature Reviews Disease Primers. 2015;1 (15003). Macmillan Publishers Limited. http://dx.doi.org/10.1038/nrdp.2015.3.
- Barker C, Postow M. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;5(1):986-997.
- Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32): 5233-5239.
Citation
C W, S C, J Y, K W, HB M. Total body irradiation: A practical review. Appl Radiat Oncol. 2016;(2):11-17.
June 4, 2016