Time-Driven Activity-Based Costing of CT-Guided vs MR-Guided Prostate SBRT
Images

Abstract
Background and Purpose: Stereotactic body radiation therapy (SBRT) has become a standard-of-care option for localized prostate cancer. While prostate SBRT has traditionally been delivered using computed-tomography-guided radiation therapy (CTgRT), MR-imaging-guided radiation therapy (MRgRT) is now available. MRgRT offers real-time soft-tissue visualization and ease of adaptive planning, obviating the need for fiducial markers, and potentially allowing for smaller planning target volume (PTV) margins. Although prior studies have focused on evaluating the cost-effectiveness of MRgRT vs CTgRT from a payor perspective, the difference in provider costs to deliver such treatments remains unknown. This study thus used time-driven activity-based costing (TDABC) to determine the difference in provider resources consumed by delivering prostate SBRT via MRgRT vs CTgRT.
Methods: Data was collected from a single academic institution where prostate SBRT is routinely performed using both CTgRT and MRgRT. Five-fraction SBRT (40 Gy total dose) was assumed to be delivered through volumetric-modulated arc therapy for CTgRT patients, and through step-and-shoot, fixed-gantry intensity-modulated radiation therapy for MRgRT patients. Process maps were constructed for each portion of the radiation delivery process via interviews/surveys with departmental personnel and by measuring CTgRT and MRgRT treatment times. Prior to simulation, only CTgRT patients underwent placement of three gold fiducial markers. Personnel capacity cost rates were calculated by dividing total personnel costs by the annual minutes worked by a given personnel. Equipment costs included both an annualized purchase price and annual maintenance costs. Ul timately, the total costs of care encompassing personnel, space/equipment, and materials were aggregated across the entire chain of care for both CTgRT and MRgRT patients in a base case.
Results: Direct costs associated with delivering a 5-fraction course of prostate SBRT were $1,497 higher with MRgRT than with CTgRT – comprised of personnel costs ($210 higher with MRgRT), space/equipment ($1,542 higher with MRgRT), and materials ($255 higher with CTgRT). Only CTgRT patients underwent fiducial placement, which accounted for $591. MRgRT patients were assumed to undergo both CT simulation (for electron density calculation) and MRI simulation, with the former accounting for $168. Mean time spent by patients in the treatment vault per fraction was 20 minutes (range 15-26 minutes) for CTgRT, and 31 minutes (range 30-34 minutes) for MRgRT. Patient time spent during fiducial placement (CTgRT only) was 60 minutes. Modifying the number of fractions treated would result in the cost difference of $1,497 (5 fractions) changing to $441 (1 fraction) or to $2,025 (7 fractions).
Conclusion: This study provides an approximate comparison of the direct resources required for a radiation oncology provider to deliver prostate SBRT with CTgRT vs MRgRT. We await findings from the currently accruing phase III MIRAGE trial, which is comparing these modalities, and will subsequently measure acute and late genitourinary/gastrointestinal (GU/GI) toxicities, temporal change in quality-of-life outcomes, and 5-year biochemical, recurrence-free survival. Results from studies comparing the efficacy and safety of MRgRT vs CTgRT will ultimately allow us to put this cost difference into context.
Ultrahypofractionation or stereotactic body radiation therapy (SBRT) has now become a standard-of-care option for localized prostate cancer.1 While SBRT has traditionally been delivered using linear accelerators (linacs) employing computed-tomography-guided radiation therapy (CTgRT), recent technological advances have allowed for MR-imaging-guided radiation therapy (MRgRT) to treat patients with radiation. Initially pioneered for use in thoracic and gastrointestinal malignancies, MRgRT has recently been highlighted in prostate cancer,2,3 offering several advantages including real-time, soft-tissue visualization and ease of adaptive planning, obviating the need for fiducial markers, and potentially allowing for smaller planning target volume (PTV) margins.
Although prior studies have focused on evaluating the cost-effectiveness of MRgRT vs CTgRT from a payor perspective,4,5 this study aimed to determine the difference in provider resources consumed by delivering prostate SBRT via MRgRT vs CTgRT. This study used time-driven activity-based costing (TDABC), an accounting technique conceptualized by Kaplan and Anderson in 2004,6 to quantify the overall personnel, space/equipment, and material costs associated with SBRT delivered with MRgRT vs with CTgRT. Accounting for numerous processes and variation in key inputs, TDABC lends itself well to radiation oncology, where in recent years it has increasingly been utilized – including notable studies in prostate cancer.7,8 In addition to quantifying resources utilized at discrete steps, the granular nature of TDABC may also lead to insights that may be used to improve care processes and gain efficiencies.
Methods
Building Process Maps to Define the Intervention
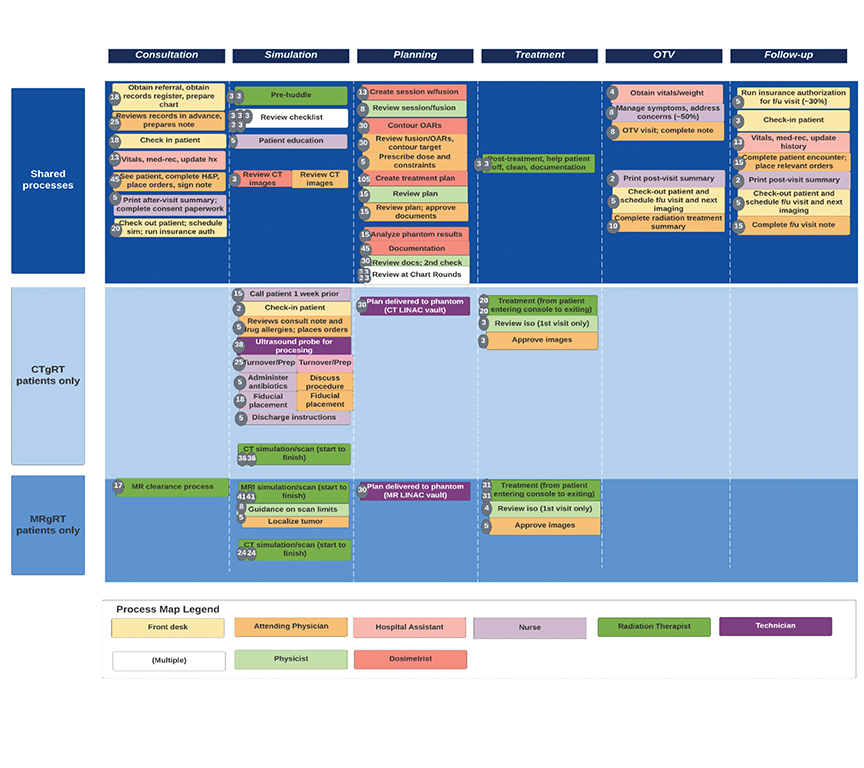
To inform this TDABC model, data were collected from a single academic institution where prostate SBRT is routinely performed using both CTgRT and MRgRT. Process maps were initially constructed for each portion of the radiation delivery process: initial consultation, simulation, treatment planning, treatment delivery over 5 SBRT fractions, 1 on-treatment visit (OTV), and 1 follow-up visit. The amount of time spent during individual processes of care was obtained by interviews/surveys with departmental personnel (physicians, nurses, dosimetrists, physicists, front office personnel, and radiation therapists), with the exception of CTgRT and MRgRT treatment times, which were measured from patients undergoing prostate SBRT from April 2021 to June 2021. A map overlooking the entire flow of care, including notable differences between CTgRT vs MRgRT, is seen in Figure 1.
Technology Utilized
Prior to simulation, CTgRT patients underwent placement of 3 gold fiducial markers by a radiation oncologist that was done in a perineal fashion using transrectal ultrasound, lithotomy position, and local lidocaine block – all in the outpatient setting. CTgRT patients were then assumed to be treated on TrueBeam STx (Varian) with on-table position management involving the ExacTrac patient positioning system (BrainLab), which utilizes kV orthogonal x-rays with fiducial matching. Treatment was performed via volumetric-modulated arc therapy (VMAT). During treatment, CTgRT patients initially underwent ExacTrac (matched to fiducials) and cone-beam CT (CBCT) (to ensure appropriate bladder filling and rectum emptiness) prior to the first arc, with ExacTrac only performed between the first and second arc.
MRgRT patients were assumed to be treated on MRIdian linac (ViewRay), a platform that integrates a linac with split-magnet MRI technology and provides continuous soft-tissue imaging during treatment. Patients were treated with step-and-shoot, fixed-gantry intensity-modulated radiation therapy (IMRT) involving 10-17 beams. Image-guidance was performed by fine tuning localization with MRI to the prostate itself. Our institutional protocol did not routinely utilize adaptive planning in the treatment of localized prostate cancer; therefore, estimates pertaining to the additional time and resources for adaptive planning were not included.
Both groups of patients were to receive prostate SBRT in 40 Gy over 5 fractions, every other day, approximately over 1.5 weeks. Preparation prior to simulation and treatment in both groups included obtaining full bladder (patients asked to fully void and then drink 16-24 ounces of water approximately 30 minutes before simulation/treatment), and empty rectum (obtained by using two fleet enemas before treatment). If simulation/treatment was scheduled prior to 2 pm, the patient was instructed to do one enema the night before, and another enema the morning of treatment upon waking up. If simulation/treatment was instead scheduled after 2 pm, the patient was instructed to do an enema that day upon waking up, and another enema at 12 pm.
Estimating the Cost of Supplying Patient Care Resources
Personnel capacity cost rates (CCRs) were calculated by dividing total personnel costs (including salary, bonuses, benefits, cost of administrative support, malpractice insurance for physicians, educational funds, information technology, and office expenses) by the annual minutes worked by a given personnel member. These estimates were obtained from the department chief financial officer. Ultimately personnel CCRs were found to be $5.16/minute for attending radiation oncologists, $1.32/minute for technologists, $2.56/minute for physicists, $2.27/minute for dosimetrists, $2.11/minute for radiation therapists, $2.42/minute for nurses, $1.09/minute for hospital assistants, $0.97/minute for front desk staff, and $0.70/minute for environmental services staff members.
The cost of equipment included both the average sales price amortized over a useful life of 10 years, as well as annual maintenance costs. The combined sales price of TrueBeam STx with ExacTrac was estimated to be $4,750,000, with estimated annual maintenance costs of $417,500. Sales price for MRIdian linac was estimated to be $7,800,000, with estimated annual maintenance costs of $550,000. Each of these estimates was provided by company representatives as typical sales prices; actual sales prices vary and are subject to change.
Space costs were made on a dollar per square foot ($/sq ft) basis. New construction costs based on institutional estimates were $1,000/sq ft for the CTgRT linac vault, $1,265/sq ft for the MRgRT linac vault (higher due to additional radiofrequency shielding and considerations involving a superconductor magnet with helium), and $420/sq ft for all other spaces; useful life of all spaces was assumed to be 25 years. All space and equipment were assumed to be available for clinical use 5 days per week (except for 10 holidays per year and 2 days per year for maintenance); during each working day, all linacs and the CT simulator were available for clinical use for 9.5 hours (machine-specific quality assurance [QA] assumed to occur outside this window) and all other spaces made available 8 hours per day.
The overwhelming majority of materials costs incurred were related to fiducial placement (associated with CTgRT delivery only), and were obtained from the lead nurse overseeing such procedures. The invoice cost of a 3-pack of gold fiducial markers was $210 each. Additionally, per-patient material costs associated with room turnover, draping the patient and preparing a sterile field, and medicating the patient were also included. Both groups of patients were assumed to have not undergone hydrogel placement.
Also included in this estimate was machine-specific QA for each linac computed by amortizing these costs across the percentage of a linac’s clinically available minutes spent on an individual treatment. The CT-guided linac and MR-guided linac were estimated to have daily QA of 20 minutes/day vs 40 minutes/day, monthly QA of 240 minutes/month vs 360 minutes/month, and yearly QA of 900 minutes/year vs 1,380 minutes/year, respectively.
Calculating Total Cost of Care
Ultimately, the total costs of care encompassing personnel, space/equipment, and materials were aggregated across the entire chain of care for both CTgRT and MRgRT patients in a base case. A synopsis of major assumptions used in calculating CTgRT and MRgRT costs is presented in Table 1. Additional sensitivity analysis is found in subsequent sections of the manuscript.
Results
Base Case Scenario
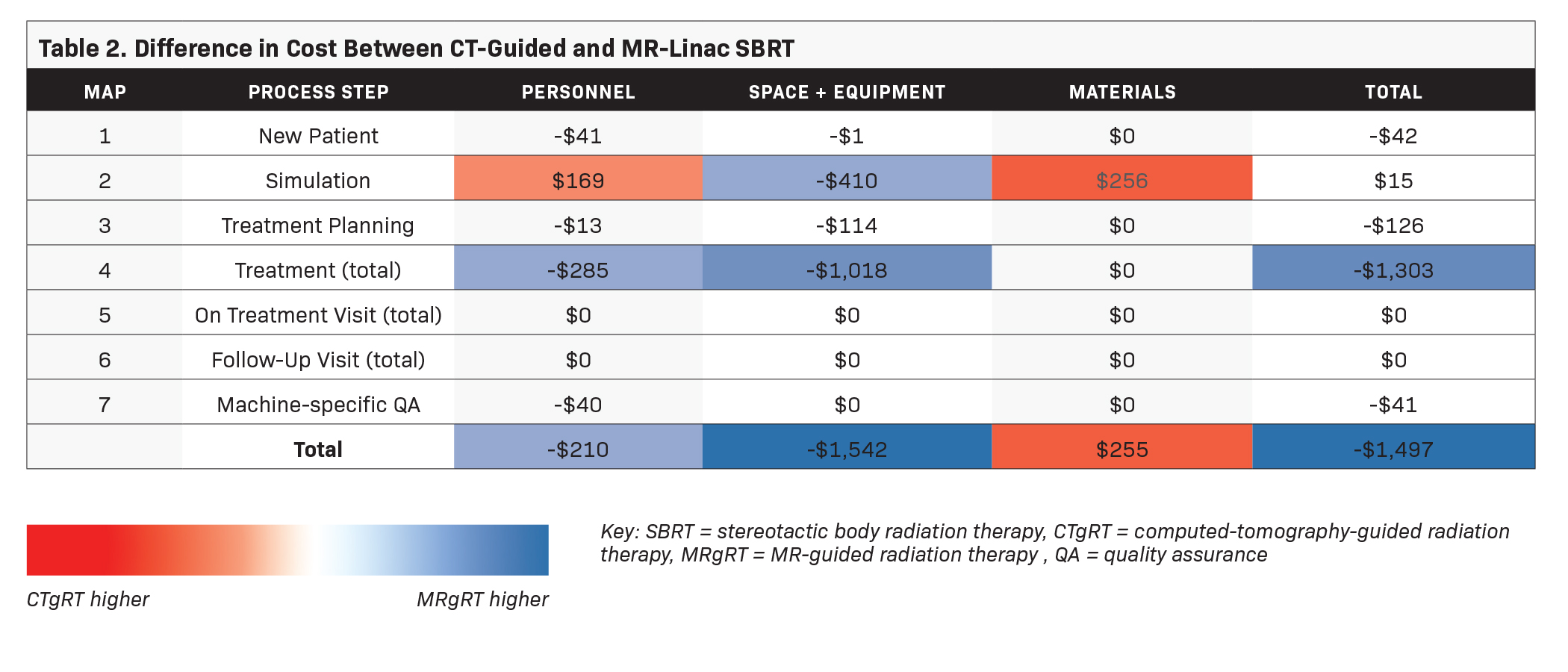
Given the baseline models as discussed above, the direct costs associated with delivering a 5-fraction course of prostate SBRT were $1,497 higher with MRgRT than with CTgRT – comprised of personnel ($210 higher with MRgRT), space/equipment ($1,542 higher with MRgRT), and materials ($255 higher with CTgRT). Differences in costs are broken down by phase of care (Table 2), with the largest differences seen in treatment delivery ($1,303 higher for MRgRT).
At simulation, both personnel and materials costs were higher with CTgRT ($169 and $256, respectively) than with MRgRT given the need for fiducial placement (only necessary for CTgRT and accounting for $591 overall). During simulation, however, space/equipment costs were $410 higher in MRgRT given the need for CT simulation to be performed (for electron density calculations) in addition to the utilization of the high-cost MRI linac vault for simulation scans.
During treatment delivery, MRgRT resulted in $1,303 higher costs per course mainly due to $1,018 higher space/equipment costs from both increased time in the vault (171 minutes for MRgRT vs 115 minutes for CTgRT during treatment delivery), as well as higher space/equipment CCR ($9.62/minute vs $6.43/minute). When estimating the time spent from a patient entering to exiting the room (mean 20 minutes [range 15-26 minutes] for CTgRT-based treatment on 5 patient encounters; mean 31 minutes [range 30 to 34 minutes] for MRgRT-based on 6 patient encounters) these estimates intentionally excluded patients who required additional waiting in the room for bladder filling. For MRgRT, the possibility of adaptive treatment was not included in this analysis.
Regarding patient time, CTgRT patients spent 30 additional minutes during simulation largely due to fiducial placement, which occupied 60 minutes (excluding the variable wait time between fiducial placement and same-day simulation). This was only partially offset by the dual CT and MRI simulation scans that MRgRT patients underwent. During treatment delivery, MRgRT patients spent 56 more minutes across the entire treatment course.
Additional Sensitivity Analyses
Instead of performing SBRT over 5 fractions, the study also compared how decreasing treatment to a single fraction (as in PROSINT)9 or increasing to 7 fractions (as in HYPO-RT-PC)10,11 would influence costs for both modalities. The overall cost increase from CTgRT to MRgRT would change from $1,497 (5 fractions) to $441 (1 fraction) or to $2,025 (7 fractions).
By decreasing the amount of time machines were clinically available by 20%, the cost difference from CTgRT to MRgRT went from $1,497 to $1,893; when increasing clinically available time by 20%, the cost difference decreased to $1,233. Decreasing the list price for each linac 20%, the CTgRT-MRgRT cost difference declined from $1,497 to $1,328. By decreasing the list price for only MRgRT by 20%, the cost difference declined from $1,497 to $1,231.
Currently, CT simulation is still performed for MRgRT patients to aid with electron density calculations. However, if synthetic CT images were used instead – similar to a process outlined in MR-OPERA12 – this would result in savings of $168.
Discussion
This study provides an approximate comparison of the direct resources required for a radiation oncology provider to deliver prostate SBRT with CTgRT vs MRgRT. For context, this $1,497 increase in direct costs from utilizing MRgRT for 5-fraction prostate SBRT instead of CTgRT is comparable to a $1,316 increase seen with MRgRT in an analysis previously conducted for patients with unresectable hepatocellular carcinoma receiving liver SBRT.13
Notably, this analysis does not include radiology resources utilized in obtaining a diagnostic prostate MRI that may be ordered for planning purposes in CTgRT patients. While many CTgRT and MRgRT patients alike may receive diagnostic MRI at initial staging, a subset of CTgRT patients undergoing neoadjuvant androgen deprivation therapy will require an additional MRI (for planning purposes) around the time of CT planning to account for prostate size. Although TDABC estimates from this step are not available, 2021 Medicare Physician Fee Schedule reimbursements total approximately $462 for prostate MRI – with national payment amounts (nonfacility price) for CPT codes 72197 (MRI pelvis with-without contrast) and 76377 (3D rendering with interpretation) at $389 and $73, respectively.
Also not included in this analysis is the possibility of adaptive planning. While a couple of studies involving MRgRT in prostate cancer have utilized adaptive planning,14,15 the incremental benefit, if any, of such an approach has not yet been elucidated. Because our institution does not routinely utilize adaptive planning for MRgRT, the nontrivial increased time and resources associated with such an effort were not included.
Lastly, not included in this analysis is the placement of a hydrogel spacer. While randomized data have shown placement of a rectal spacer resulting in improved rectal toxicity and sexual function,16,17 it is not covered by all payors and may also result in rare grade 3 toxicity (including rectum perforation and urethral damage).18 As a result, its utilization often depends on physician experience, patient preference, and clinical factors. Although our analysis did not account for spacer hydrogel placement, it is worth noting that the cost differential between a CTgRT patient receiving fiducials plus hydrogel vs an MRgRT patient receiving hydrogel alone would be significantly smaller than $591 (the cost currently attributed to doing fiducial placement alone in CTgRT patients). Because most steps are shared in a combined fiducial plus hydrogel placement, the additional cost from placing fiducials in this setting mainly comes from materials costs of the fiducials themselves.
Although this study focuses exclusively on the resources associated with processes, personnel, space/equipment, and materials involved in performing prostate SBRT with CTgRT vs MRgRT, we currently await data comparing the safety/efficacy of the two modalities. While single-arm prospective data by Tetar et al has illustrated a favorable safety profile with MRgRT prostate SBRT (no grade 3-plus toxicity reported; symptoms returning to baseline by 12 months),2 the currently accruing phase III MIRAGE trial aims to formally compare these modalities in a randomized fashion, and will subsequently measure acute and late GU/GI toxicities, temporal change in quality-of-life outcomes, and 5-year biochemical recurrence-free survival.3 While real-time image guidance may allow for smaller PTV margins with MRgRT, it is unclear how this will compare to the difference in dosimetry achieved by VMAT with CTgRT vs step-and-shoot IMRT with MRgRT.
Given comorbidities and clinical situations, it is likely that certain patients may be suitable for one modality. For example, patients with extreme claustrophobia or with nonpacemaker-compatible implanted devices may not be suitable candidates for MRgRT. On the other hand, patients with an excessive bleeding risk or who cannot easily come off anticoagulants may not be suitable for CTgRT given the need for fiducial placement.
Finally, one must acknowledge the following caveats to the analysis when interpreting this study’s results, and especially when extrapolating findings to other centers. First, the data used to inform process times, personnel costs, and materials costs comes from a single academic department, where protocols and processes may vary compared with other institutions. For example, while our institution utilized fiducials for CTgRT SBRT delivery, this practice is not universal, as 27% of SBRT patients treated in PACE-B did not receive fiducials.19 In addition, our institution utilized both CBCT and orthogonal kV x-rays before treatment as well as orthogonal kV x-rays during treatment, whereas other centers may have only used CBCT prior to treatment – thereby resulting in lower treatment times. Second, the equipment costs used in this analysis were taken from sales representatives and may be subject to variation depending on specific contract agreements. Third, when accounting for different fractionation regimens (eg, 1 fraction or 7 fractions vs 5 fractions), the approximate cost per fraction was kept constant and did not explicitly account for the variable length of treatment time depending on nominal dose delivered.
Conclusion
In conclusion, the base case of this TDABC analysis estimates $1,497 in increased direct costs utilized by delivering prostate SBRT with MRgRT instead of CTgRT, although as seen in sensitivity analyses above, modifications to key model inputs may change this result. Results from studies comparing the efficacy and safety between MRgRT vs CTgRT will ultimately allow us to put this cost difference into context.
References
- National Comprehensive Cancer Network. Prostate Cancer (Version 2.2021). Accessed July 1, 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- Tetar SU, Bruynzeel AME, Oei SS, et al. Magnetic resonance-guided stereotactic radiotherapy for localized prostate cancer: final results on patient-reported outcomes of a prospective phase 2 study. Eur Urol Oncol. 2020.
- Ma TM, Lamb JM, Casado M, et al. Magnetic resonance imaging-guided stereotactic body radiotherapy for prostate cancer (mirage): a phase III randomized trial. BMC Cancer. 2021;21:538.
- Schumacher LD, Dal Pra A, Hoffe SE, et al. Toxicity reduction required for MRI-guided radiotherapy to be cost-effective in the treatment of localized prostate cancer. Br J Radiol. 2020;93:20200028.
- Hehakaya C, van der Voort van Zyp JRN, Vanneste BGL, et al. Early health economic analysis of 1.5 T MRI -guided radiotherapy for localized prostate cancer: decision analytic modelling. Radiother Oncol. 2021;161:74-82.
- Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82:131-8,150.
- Laviana AA, Ilg AM, Veruttipong D, et al. Utilizing time-driven activity-based costing to understand the short- and long-term costs of treating localized, low-risk prostate cancer. Cancer. 2016;122:447-455
- Dutta SW, Bauer-Nilsen K, Sanders JC, et al. Time-driven activity-based cost comparison of prostate cancer brachytherapy and intensity-modulated radiation therapy. Brachytherapy. 2018;17:556-563.
- Greco C, Pares O, Pimentel N, et al. Safety and efficacy of virtual prostatectomy with single-dose radiotherapy in patients with intermediate-risk prostate cancer: results from the prosint phase 2 randomized clinical trial. JAMA Oncol. 2021;7:700-708.
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the hypo-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385-395.
- Fransson P, Nilsson P, Gunnlaugsson A, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer (HYPO-RT-PC): patient-reported quality-of-life outcomes of a randomised, controlled, non-inferiority, phase 3 trial. Lancet Oncol. 2021;22:235-245.
- Persson E, Gustafsson C, Nordstrom F, et al. MR-opera: a multicenter/multivendor validation of magnetic resonance imaging-only prostate treatment planning using synthetic computed tomography images. Int J Radiat Oncol Biol Phys. 2017;99:692-700.
- Parikh NR, Lee PP, Raman SS, et al. Time-driven activity-based costing comparison of ct-guided versus mr-guided sbrt. JCO Oncol Pract. 2020;16:e1378-e1385.
- Alongi F, Rigo M, Figlia V, et al. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: Feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat Oncol. 2020;15:69.
- Bruynzeel AME, Tetar SU, Oei SS, et al. A prospective single-arm phase 2 study of stereotactic magnetic resonance guided adaptive radiation therapy for prostate cancer: early toxicity results. Int J Radiat Oncol Biol Phys. 2019;105:1086-1094.
- Hamstra DA, Mariados N, Sylvester J, et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: secondary analysis of a phase 3 trial. Pract Radiat Oncol. 2018;8:e7-e15.
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976-985.
- Schorghofer A, Drerup M, Kunit T, et al. Rectum-spacer related acute toxicity – endoscopy results of 403 prostate cancer patients after implantation of gel or balloon spacers. Radiat Oncol. 2019;14:47.
- Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (pace-b): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20:1531-1543.
Citation
NR P, MA C, P P, K K, L Z, TM M, ML S, M C, AC R, J L, AU K. Time-Driven Activity-Based Costing of CT-Guided vs MR-Guided Prostate SBRT . Appl Radiat Oncol. 2021;(3):33-40.
October 5, 2021