The role of image-guided brachytherapy in the treatment of gynecologic malignancies
Images
Gynecologic malignancies, including uterine, cervical, ovarian, vulvar and vaginal cancers are diagnosed in approximately 71,500 U.S. women each year, 26,500 of whom will die from their disease.1 The presentation and treatment paradigm for each of these cancers is somewhat distinct, and radiation therapy represents a mainstay of treatment for many patients diagnosed with a gynecologic malignancy — especially those with cancers of the cervix, uterus, vulva or vagina.
Although external-beam radiation therapy (EBRT) techniques are generally employed to treat microscopic disease within the pelvis, the dose required to definitively treat these tumors often exceeds the normal tissue tolerance of the small bowel and other organs in the pelvis, making treatment with EBRT alone a poor choice. Fortunately, the anatomy of the female genital tract is predisposed to the use of intracavitary or interstitial brachytherapy techniques, allowing for the delivery of higher doses of radiation therapy to primary tumors arising within the nonadnexal genital organs and sparing toxicity to surrounding normal tissues.
Brachytherapy has been used for treating malignancies since 1901 — shortly after the discovery of radiation by Henri Becquerel. Intracavitary and interstitial techniques were widely used for a range of malignancies in the early and middle part of the 20th century, but fell out of favor for many cancer types due to improvements in teletherapy technology and its ease of delivery. External-beam planning and delivery techniques continued to improve over time, and since the 1990s, many patients in the United States (and largely worldwide) have been treated using computed tomography (CT)-based planning techniques. The ability to fuse diagnostic imaging (eg, MRI or positron emission tomography [PET]) to a planning scan to help delineate target volumes and organs at risk (OAR); and image guidance, which allows for more accurate patient setups and, thus, smaller target margins and better sparing of normal tissues; can potentially increase tumor control while decreasing normal tissue toxicity. Although brachytherapy stayed in the 2-dimensional “dark ages” longer than teletherapy, image-guided brachytherapy (IGBT) techniques using CT, MRI or ultrasound have been described since the early 2000s. These techniques are increasingly embraced by the radiation oncology community for the same reasons as image-guided teletherapy, namely improved target delineation and planning, potentially improving local control and normal tissue sparing. Here we describe the role of IGBT in the treatment of cervical, uterine and vaginal cancers.
Cancer of the cervix
Cervical cancer is the third most common cancer in women worldwide. In patients with locally advanced presentations, the standard treatment paradigm consists of chemoradiation therapy — external-beam radiation therapy to the pelvis +/- para-aortics (typically 45-50.4 Gy) with concurrent weekly cisplatin chemotherapy, followed by a boost dose of radiation delivered to the primary cervical tumor.2,3 This boost has historically been delivered via brachytherapy, and the use of brachytherapy in these patients has been shown to confer a survival benefit over treatment with external-beam radiation treatment alone.4,5 The boost is frequently delivered after completion of the initial pelvic RT to allow for tumor shrinkage and improved applicator geometry. High-dose-rate (HDR) brachytherapy has been shown to be noninferior to traditional low-dose-rate (LDR) techniques with respect to local control outcomes, with the added advantage of outpatient treatment (mitigating the need for inpatient stays and prolonged bedrest) and decreased exposure of ionizing radiation to healthcare personnel.6 HDR brachytherapy is typically delivered in 5 fractions of 5-6 Gy per fraction given at least 72 hours apart.7 Due to the need for multiple insertions of the applicator system with HDR brachytherapy and the potential changes in applicator geometry and/or pelvic anatomy (changes in bladder and rectal filling, and uterine position) between fractions that may affect dose distributions to target volumes (and therefore affect local control and toxicity outcomes), the use of 3-dimensional IGBT has been most extensively studied in this setting.
Advantages of 3-dimensional IGBT planning
Historically, treatment planning has been performed using 2-dimensional techniques with the dose prescribed to a modification of the classical Manchester system point A for target coverage and specified points for normal tissues on conventional radiographs. This technique follows a “one-size-fits-all” approach and does not allow for individualized dose distribution based on patient-specific factors. Three-dimensional IGBT allows a practitioner to modify dose distributions based on a patient’s individual anatomy and tumor response, typically using CT and/or MRI. Ultrasound has also been used, but will not be discussed here. The Vienna group pioneered the use of IGBT (using MRI based on its superior soft tissue contrast compared to CT imaging) for cervical cancer in the early 2000s with the goal of improving target coverage — especially in bulkier and more locally advanced presentations — and decreasing normal tissue toxicity by better understanding dose distributions to OARs since dose volume histogram (DVH) information from a brachytherapy insertion could now be obtained.8-12
Since then, several centers have compared 2-dimensional vs. 3-dimensional planning in dosimetric studies and have shown improved cervical tumor coverage and decreased dose to critical normal tissues with 3-dimensional planning. One study from the University of Alabama, Birmingham, revealed that prescription to point A allowed for excellent GTV coverage for earlier stage tumors, but overestimated tumor coverage in more locally advanced cases (IB1 98.5%, IB2 89.5%, IIB 79.5%, and IIIB 59.5%).13 Other prospective studies from MD Anderson, Korea and Vienna have shown that standard specified normal tissue points (defined by ICRU 38) can underestimate the dose to the OAR.14-16 These studies have also helped to obtain valuable correlative data on normal tissue dose and long-term toxicity to better define appropriate and clinically relevant normal tissue constraints with modern IGBT (see recommended guidelines).
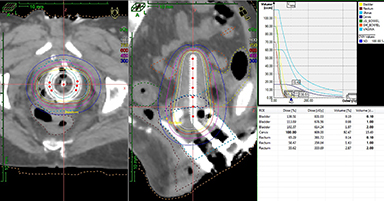
Other potential advantages of 3-dimensional-based planning include (1) verification of tandem placement in the uterine cavity and decreasing the risk of treating a patient with a uterine perforation; (2) a better understanding of the doses delivered to other normal tissues at risk, especially the small bowel, and the potential to spare dose to these organs (Figure 1); (3) the ability to use more combined intracavitary/interstitial techniques (Vienna applicator) for locally advanced disease to achieve better coverage of gross disease while sparing normal tissue; and (4) optimized target coverage and normal tissue dose with adaptive replanning based on tumor response.17
Clinical outcomes with 3-dimensional IGBT
The emerging data for improved outcomes with 3-dimensional IGBT is promising. Georg et al from the University of Vienna published their initial experience of patients with IB-IVA cervical cancer treated with MRI-based IGBT as defined by the Groupe Europeen de Curietherapie/European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) guidelines.12,18 At a median follow-up of 51 months, local control was achieved in 95% (134 of 141 patients), an improvement over historic controls. Negative prognostic factors for local control included patients with a large tumor (> 5 cm) both at the time of diagnosis and implant (local recurrence 35%); however, significant tumor regression (< 5 cm tumor) after initial pelvic chemo-RT was a positive prognostic factor, with a local recurrence rate of 10.9%. A clear relationship between cumulative rectal dose and grade 2-4 late toxicity was also reported (D2cc: 67 Gy - 5%, 78 Gy -10%, 90 Gy - 20%) by the same group, indicating that image guidance to help lower rectal dose ultimately decreases late toxicity.19 Interestingly, no correlation was found between total bladder dose and late toxicity.
Creutzberg et al reported improved survival outcomes in patients with IB-IVA disease treated with MR-based IGBT at Leiden University.20 Comparing 83 patients treated with IGBT to 43 historical patients treated with 2-dimensional techniques, they reported a 3-year overall survival of 86% vs. 51% (p = 0.001) and complete remission in 98.8% vs. 83.7% (p < 0.01) favoring the IGBT group. Grade 3-4 toxicities reported at 3 years also showed a trend toward improvement with IGBT (8.4% vs. 15.4%, p = 0.06). This has also been corroborated in studies out of North America. The University of Pittsburgh published its experience earlier this year with 128 patients with IB1-IVA cervical cancer treated with a hybrid MR/CT-based IGBT technique (MR required for at least 1 fraction) after pelvic RT.21 At 24.4 months follow-up, estimated 2-year outcomes were: local control 91.6%, disease-free survival 81.8%, and cancer-specific survival 87.6%. The 2-year actuarial rate of late grade 3+ toxicity was 0.9%. Predictors of local failure were adenocarcinoma histology and 3-month clinical response; importantly, a cumulative dose to the HR-CTV (dose to 90% of treatment volume, D90) of > to 84 Gy in equivalent 2 Gy doses (EQD2) in adenocarcinoma was associated with improved local control (2-year LC 100% vs. 54.5%).
Despite excellent MR-based IGBT outcomes, unfortunately, many centers lack ready access to MRI scanners for MR-based IGBT, making widespread adoption of IGBT and GEC-ESTRO-based contouring challenging. A prospective international cooperative group trial compared CT-based IGBT to MR-based IGBT planning and revealed similar HR-CTV volume, height, and thickness contour measurements between the 2 imaging modalities, as well as similar DVH values for OARs.22 HR-CTV width contours differed between the 2 modalities leading to significant differences in the volume treated to the prescription dose or greater (MRI 96% vs. CT 86%, p = 0.01) and D90 (MRI 8.7% vs. CT 6.7%, p < 0.01). However, clinical experience from Addenbrook with CT-based IGBT using GEC-ESTRO guidelines compared to 2-dimensional-based planning still showed a 20% improvement in local control (p = 0.04) favoring IGBT.23 This study shows promise in improving outcomes for patients with IGBT planning in centers that only have CT imaging available.
Challenges with 3-dimensional IGBT
The biggest potential challenges of 3-dimensional IGBT planning include (1) the cost of software/hardware needed to perform treatment planning; (2) increased use of expensive imaging studies and resources for each patient; (3) dosimetric uncertainties both inter- and intrafractionally, given the anatomic variation of normal tissues in the pelvis (ie, bladder/rectal filling) and the potential movement of an applicator between image acquisition for treatment planning and radiation delivery; and (4) lack of experienced personnel for MR interpretation of tumor response and subsequent contouring of GTV and CTVs for treatment planning.17 A recently published analysis, however, concluded that 3-dimensional IGBT for locally advanced cervical cancer is cost-effective compared to 2-dimensional treatment.24
Guidelines for planning with 3-dimensional techniques
Image guidance to verify applicator position and to rule out uterine perforation prior to treatment delivery is generally recommended. Also recommended is following the GEC-ESTRO and American Brachytherapy Society (ABS) published guidelines for IGBT for 3-dimensional IGBT treatment planning, including delineation of target volumes and OARs, and defining appropriate dosing and DVH constraints.8,25-29 Both GEC-ESTRO and ABS guidelines center on MRI-based planning due to its superior soft-tissue contrast compared to CT-based imaging, but can be translated for use with CT, with improved outcomes over 2-dimensional planning.30 If no MR planning is available, it is recommended that patients undergo repeat MR imaging after completing pelvic RT to aid in CT-based planning. Hybrid approaches have also been shown to be feasible with the first brachytherapy fraction MRI-planned and subsequent fractions CT-planned.31-33 Image-based planning using point A is common; however, Beriwal et al have shown in dosimetric studies that prescription to point A generally leads to decreased D90 coverage of HR-CTV compared to volume-based planning.34
Cancer of the endometrium
Endometrial cancer is the most common gynecologic cancer in the United States. Brachytherapy is most often used in this disease in (1) the adjuvant setting to decrease risk of recurrence in the vaginal cuff for early stage disease (depending on risk factors after total extrafascial hysterectomy, bilateral salpingo-oophorectomy, and lymph node dissection of the pelvic and para-aortic nodes, if indicated), (2) the definitive setting for patients with medically inoperable disease, and (3) the salvage setting for patients with recurrent disease in the vaginal cuff. Less data exists for the use of 3-dimensional IGBT in the treatment of endometrial cancer compared to cervical cancer (and is summarized below), but many of the same principles apply — namely the potential for improved local control and decreased normal tissue toxicity when targets and OARS can be visualized and more clearly defined on 3-dimensional images, at the potential expense of increased costs and resources. No published guidelines exist for 3-dimensional IGBT for endometrial cancer.
Adjuvant treatment to the vaginal cuff
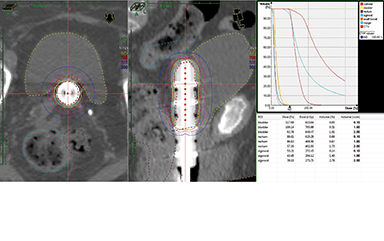
Vaginal cylinder treatment as adjuvant therapy in early stage disease is the most common indication for the use of brachytherapy in endometrial cancer.35,36 Two-dimensional imaging with anterior-posterior and lateral radiographs is used to verify cylinder placement and for treatment planning. At least 2 series have shown that 3-dimensional IGBT may allow for better target coverage by identifying air gaps after cylinder placement, which would otherwise reduce vaginal mucosal dose if not corrected.37,38 Other potential advantages may include identification of vaginal cuff perforation by the cylinder, as well as DVH data for normal tissues (Figure 2) with the possibility to correlate to late toxicities and better define normal tissue constraints. Very limited data exists for this application though, and further studies are needed to better understand whether 3-dimensional IGBT can improve outcomes in the adjuvant setting.
Definitive treatment for medically inoperable patients
The University of Pittsburgh group recently published results using high-dose-rate 3-dimensional IGBT with MRI or CT-based planning in 38 medically inoperable stage I patients treated with IGBT alone (37.5 Gy in 5-6 fractions) or EBRT (45 Gy in 25 fractions) in combination with IGBT (25 Gy in 4-5 fractions).39 Dose was prescribed to a CTV including the entire uterus, cervix and upper 1-2 cm of vagina; a GTV was also defined in patients undergoing MRI. Two year local control was 90.6% and overall survival was 94.4% with no grade 2-5 toxicities. GTV doses (D90 EQD2) ranged from 138-233 Gy, which was felt to account for the very high local control. Based on results, the authors conclude that 3-dimensional IGBT is feasible and potentially beneficial in this setting. The Vienna group achieved similar outcomes using a modified Heyman technique.39
Salvage treatment for vaginal cuff recurrences
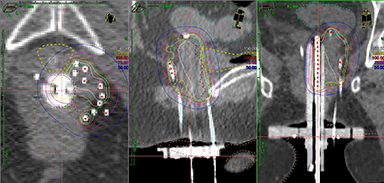
Viswanathan et al evaluated the outcomes of patients treated with MR- or CT-guided salvage interstitial brachytherapy in 44 patients with vaginal cuff recurrences, 13 of whom had received prior RT.41 At 2 years, local failure was 4% in patients with no prior RT and 39% in patients with prior RT, likely due to the lower doses achieved in patients undergoing re-irradiation (mean D90 EQD2 < 70 Gy vs. > 70 Gy if no prior RT). Grade 3 late toxicity was noted in 4 patients, only 1 of whom had not received prior RT. The authors conclude that 3-dimensional IGBT results in excellent local control and minimal toxicity. Similarly good outcomes were reported by Cormack et al in a prospective trial of 25 patients treated with MR-guided interstitial brachytherapy techniques. This study also reported low late toxicity rates with this method. They concluded that 3-dimensional IGBT with image guidance and planning can lead to excellent clinical outcomes and improved toxicity profiles.42 Another series from the same group using HDR interstitial therapy in women with primary or recurrent gynecologic cancers concluded that 3-dimensional IGBT helps ensure adequate tumor coverage and minimized dose (D2cc) to the rectum that can result in late late rectal complications (Figure 3).43,44 This has been corroborated by evidence from Aarhus University in Denmark.45
Cancer of the vagina
Vaginal cancer is the least common gynecologic malignancy worldwide. Treatment typically consists of an initial course of pelvic RT (with chemotherapy, if tolerated) followed by a boost to the primary tumor, often delivered via brachytherapy techniques, as extrapolated from other gynecologic malignancies.46 Given the high doses needed to achieve local control, and the high risk for potential toxicity to normal tissues (bladder, urethra, rectum), 3-dimensional IGBT may have a large benefit for these patients. The Vienna group published their outcomes in 13 patients with locally advanced vaginal cancer using MR-based IGBT.47 The mean D90 to the HR-CTV (defined based on a modification of the GEC-ESTRO guidelines) was 86 Gy. At a median follow-up of 43 months, 3-year actuarial local control was 92% and overall survival was 85%. Data with multi-channel cylinders using 3-dimensional IGBT is also promising.48,49
Conclusion
Three-dimensional IGBT is feasible and may improve clinical outcomes, including greater local control and decreased normal tissue toxicity in a wide range of gynecologic malignancies. The role of 3-dimensional IGBT is most well-defined for cervical cancer patients and is recommended for treatment planning. The benefit of 3-dimensional IGBT in other gynecologic malignancies is less clear given limited published data, and it may be harder for centers to adopt given the lack of published guidelines for contouring and planning. However, based on the available data, 3-dimensional IGBT should be considered for all patients undergoing interstitial brachytherapy or intracavitary treatment with a tandem applicator. More prospective data is needed to better define dosimetric constraints, but the use of the GEC-ESTRO guidelines for DVH evaluation is recommended.
References
- Berman ML, Daling J, Haefner HK, et al. Get the Facts About Gynecologic Cancer. Inside Knowledge (Brochure) 2015. http://www.cdc.gov/cancer/knowledge/pdf/cdc_gyn_comprehensive_brochure.pdf. Accessed August 15, 2015.
- Lanciano RM, Won M, Coia LR, Hanks GE. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: a final report of the 1973 and 1978 patterns of care studies. Int J Radiat Oncol Biol Phys. 1991;20(4):667-676.
- Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350(9077):535-540.
- Viswanathan AN, Moughan J, Small W, et al. The quality of cervical cancer brachytherapy implantation and the impact on local recurrence and disease-free survival in radiation therapy oncology group prospective trials 0116 and 0128. Int J Gynecol Cancer. 2012;22(1):123-131.
- Bandera L, La Face B, Antonioli C, et al. Survival and toxicity of radical radiotherapy (with or without brachytherapy) for FIGO stage I and II cervical cancer: a mono-institutional analysis. Eur J Gynaecol Oncol. 2014;35(2):121-127.
- Narayan K, van Dyk S, Bernshaw D, et al. Comparative study of LDR (Manchester system) and HDR image-guided conformal brachytherapy of cervical cancer: patterns of failure, late complications, and survival. Int J Radiat Oncol Biol Phys. 2009;74(5):1529-1535.
- Nag S, Erickson B, Thomadsen B, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48(1): 201-211.
- Haie-Meder C, Potter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74(3):235-245.
- Kirisits C, Potter R, Lang S, et al. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2005;62(3):901-911.
- Dimopoulos JC, Schard G, Berger D, et al. Systematic evaluation of MRI findings in different stages of treatment of cervical cancer: potential of MRI on delineation of target, pathoanatomic structures, and organs at risk. Int J Radiat Oncol Biol Phys. 2006;64(5):1380-1388.
- Potter, R., et al., 3D conformal HDR-brachy- and external beam therapy plus simultaneous cisplatin for high-risk cervical cancer: clinical experience with 3 year follow-up. Radiother Oncol. 2006;79(1):80-86.
- Potter R, Dimopoulos J, Bachtiary B, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83(2):148-155.
- Kim RY, Pareek, P. Radiography-based treatment planning compared with computed tomography (CT)-based treatment planning for intracavitary brachytherapy in cancer of the cervix: analysis of dose-volume histograms. Brachytherapy. 2003;2(4):200-206.
- Shin KH, Kim TH, Cho JK, et al. CT-guided intracavitary radiotherapy for cervical cancer: Comparison of conventional point A plan with clinical target volume-based three-dimensional plan using dose-volume parameters. Int J Radiat Oncol Biol Phys. 2006;64(1):197-204.
- Yaparpalvi R Mutylal S, Gorla GR, et al. Point vs. volumetric bladder and rectal doses in combined intracavitary-interstitial high-dose-rate brachytherapy: correlation and comparison with published Vienna applicator data. Brachytherapy. 2008;7(4):336-342.
- Onal C, Arslan G, Topkan E, et al. Comparison of conventional and CT-based planning for intracavitary brachytherapy for cervical cancer: target volume coverage and organs at risk doses. J Exp Clin Cancer Res. 2009;28:95.
- Vargo JA, Beriwal S. Image-based brachytherapy for cervical cancer. World J Clin Oncol. 2014;5(5):921-930.
- Potter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100(1):116-123.
- Georg P, Lang S, Dimopoulos JC, et al. Dose-volume histogram parameters and late side effects in magnetic resonance image-guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79(2):356-362.
- Rijkmans EC, Nout RA, Rutten IH, et al. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol. 2014;135(2):231-238.
- Gill BS, Kim H, Houser CJ, et al. MRI-guided high-dose-rate intracavitary brachytherapy for treatment of cervical cancer: the University of Pittsburgh experience. Int J Radiat Oncol Biol Phys. 2015;91(3):540-547.
- Viswanathan AN, Dimopoulos J, Kirisits C, et al. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68(2):491-498.
- Tan LT, Coles CE, Hart C, Tait, E.Clinical impact of computed tomography-based image-guided brachytherapy for cervix cancer using the tandem-ring applicator - the Addenbrooke’s experience. Clin Oncol (R Coll Radiol). 2009;21(3):175-182.
- Kim H, Rajagopalan MS, Beriwal S, et al. Cost-effectiveness analysis of 3D image-guided brachytherapy compared with 2D brachytherapy in the treatment of locally advanced cervical cancer. Brachytherapy. 2015;14(1):29-36.
- Nag S, Cardenes H, Chang S, et al. Proposed guidelines for image-based intracavitary brachytherapy for cervical carcinoma: report from Image-Guided Brachytherapy Working Group. Int J Radiat Oncol Biol Phys. 2004;60(4):1160-1172.
- Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78(1):67-77.
- Lee LJ, Das IJ, Higgins SA, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part III: low-dose-rate and pulsed-dose-rate brachytherapy. Brachytherapy. 2012;11(1):53-57.
- Viswanathan AN, Beriwal S, De Los Santos JF, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy. Brachytherapy. 2012;11(1):47-52.
- Viswanathan AN, Thomadsen B. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles. Brachytherapy. 2012;11(1):33-46.
- Viswanathan AN, Erickson B, Gaffney DK, et al. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2014;90(2):320-328.
- Beriwal S, Kim H, Coon R. Single magnetic resonance imaging vs magnetic resonance imaging/computed tomography planning in cervical cancer brachytherapy. Clin Oncol (R Coll Radiol). 2009;21(6):483-487.
- Beriwal S, Kannan N, Kim H, et al. Three-dimensional high dose rate intracavitary image-guided brachytherapy for the treatment of cervical cancer using a hybrid magnetic resonance imaging/computed tomography approach: feasibility and early results. Clin Oncol (R Coll Radiol). 2011;23(10):685-690.
- Nesvacil N, Potter N, Sturdza A, et al. Adaptive image guided brachytherapy for cervical cancer: a combined MRI-/CT-planning technique with MRI only at first fraction. Radiother Oncol. 2013;107(1):75-81.
- Kim H, Beriwal S, Houser C, Hug MS. Dosimetric analysis of 3D image-guided HDR brachytherapy planning for the treatment of cervical cancer: is point A-based dose prescription still valid in image-guided brachytherapy? Med Dosim. 2011;36(2):166-170.
- Nag S, Erickson B, Parikh S, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the endometrium. Int J Radiat Oncol Biol Phys. 2000;48(3):779-790.
- Small W, Beriwal S, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy. Brachytherapy. 2012;11(1):58-67.
- Humphrey P, Cornes P, Al-Booz H. Vaginal vault brachytherapy in endometrial cancer: verifying target coverage with image-guided applicator placement. Br J Radiol. 2013;86(1023):20120428.
- Hassouna A, Bahadur YA, and Constantinescu C. Assessment of air pockets in high-dose-rate vaginal cuff brachytherapy using cylindrical applicators. J Contemp Brachytherapy. 2014;6(3):271-275.
- Gill BS, Kim H, Houser C, et al. Image-based three-dimensional conformal brachytherapy for medically inoperable endometrial carcinoma. Brachytherapy. 2014;13(6):542-547.
- Weitmann HD, Potter R, Waldhausl C, et al. Pilot study in the treatment of endometrial carcinoma with 3D image-based high-dose-rate brachytherapy using modified Heyman packing: clinical experience and dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2005;62(2):468-478.
- Lee LJ, Damato AL, Viswanathan AN. Clinical outcomes following 3D image-guided brachytherapy for vaginal recurrence of endometrial cancer. Gynecol Oncol. 2013;131(3):586-592.
- Viswanathan AN, Cormack R, Holloway CL, et al. Magnetic resonance-guided interstitial therapy for vaginal recurrence of endometrial cancer. Int J Radiat Oncol Biol Phys. 2006;66(1):91-99.
- Lee LJ, Viswanathan AN. Predictors of toxicity after image-guided high-dose-rate interstitial brachytherapy for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2012;84(5):1192-1197.
- Lee LJ, Damato AL, Viswanathan AN. Clinical outcomes of high-dose-rate interstitial gynecologic brachytherapy using real-time CT guidance. Brachytherapy. 2013;12(4): 303-310.
- Fokdal L, Tanderup K, Nielsen SK. Image and laparoscopic guided interstitial brachytherapy for locally advanced primary or recurrent gynaecological cancer using the adaptive GEC ESTRO target concept. Radiother Oncol. 2011;100(3):473-479.
- Beriwal S, Demanes DJ, Erickson B, et al. American Brachytherapy Society consensus guidelines for interstitial brachytherapy for vaginal cancer. Brachytherapy. 2012;11(1):68-75.
- Dimopoulos JC, Schmid MP, Fidarova E. Treatment of locally advanced vaginal cancer with radiochemotherapy and magnetic resonance image-guided adaptive brachytherapy: dose-volume parameters and first clinical results. Int J Radiat Oncol Biol Phys. 2012. 82(5):1880-1888.
- Kim H, Rajagopalan, MS, Houser C, Beriwal S. Dosimetric comparison of multichannel with one single-channel vaginal cylinder for vaginal cancer treatments with high-dose-rate brachytherapy. Brachytherapy. 2014;13(3):263-267.
- Vargo JA, Kim H, Houser CJ, et al. Image-based multichannel vaginal cylinder brachytherapy for vaginal cancer. Brachytherapy. 2015;14(1):9-15.
Citation
SR A. The role of image-guided brachytherapy in the treatment of gynecologic malignancies. Appl Radiat Oncol. 2015;(4):4-10.
December 28, 2015