Technological Basis for Clinical Trials in FLASH Radiation Therapy: A Review
Images


SA-CME credits are available for this article here.
FLASH radiation therapy (RT) has shown potential to increase the therapeutic index for cancer treatment. In vivo animal studies have shown a differential response between normal tissues and tumor1-3 with improved normal tissue sparing but comparable tumor control relative to conventional RT. This phenomenon, or the “FLASH effect,” is exhibited at ultrahigh dose rates (UHDRs) of approximately 40 Gy/s or higher.1,3,4 In this review, “FLASH” is used to describe biological FLASH effects and is distinct from “ultrahigh dose rate,” pertaining simply to physical dose rate expressed in Gy/s. Although used interchangeably in the literature, this distinction is made since many complex physical parameters of radiation, beyond simply mean dose rate, may contribute to the biological effects, and is a topic under investigation.5
Studies of what we now recognize as the FLASH effect date to the 1960s,6-8 although recently interest has been rekindled. Although technological advancements in RT delivery have improved toxicities associated with radiation, this remains an ongoing hurdle in optimizing treatment efficacy. Contemporary preclinical studies continue to show a stark reduction in normal tissue toxicity with FLASH-RT compared with conventional dose-rate RT, demonstrated across multiple organ systems, including the brain,4,9-11 skin,12,13 lungs,1 and gastrointestinal tracts2 in multiple species, including mice, zebrafish, cats, and pigs.1,2,4,9-13 The clinical implications of the FLASH effect could provide major improvements in the oncologic care of patients and give rise to a new, highly impactful modality of treatment, providing the impetus for clinical translation of FLASH-RT.5
Pre-clinical FLASH-RT in animal studies has been made possible through dedicated experimental systems or modification of pre-existing RT systems, including specialized electron linear accelerators (linacs),14 proton beamlines,15 synchrotron light sources producing kilovoltage x-rays,16 and conversion of clinical linacs.17,18 Recently, the first human treatment with FLASH-RT was conducted for the treatment of a CD30+ T-cell cutaneous lymphoma lesion at Lausanne University Hospital in Switzerland.19 The institution employed an Oriatron eRT6 5.6 MeV linac (PMB ALCEN), specifically engineered for accelerating electrons for UHDR-RT. The treatment intent was to achieve equivalent tumor control while reducing skin toxicity for a patient who received 110 prior spot radiation treatments to multiple lymphoma skin lesions. Given the numerous treatments that the patient has received in the past, FLASH-RT was considered for potential toxicity reduction. Ultimately, treatment was deemed feasible and safe, with favorable outcomes for both tumor control and skin toxicity, opening the door for further clinical evaluation of FLASH-RT.
Since then, enrollment and treatment in the world’s first FLASH-RT clinical trial, FAST-01, has started at the University of Cincinnati, assessing feasibility of single fraction proton FLASH-RT for painful bone metastases.20 With additional burgeoning FLASH-RT human clinical trials underway, this review aims to cover the technological basis for FLASH-RT clinical trials and explores the modalities, treatment parameters, technical limitations, and potential indications of current UHDR-RT technologies.
Technological Basis for Active Clinical Trials in FLASH-RT
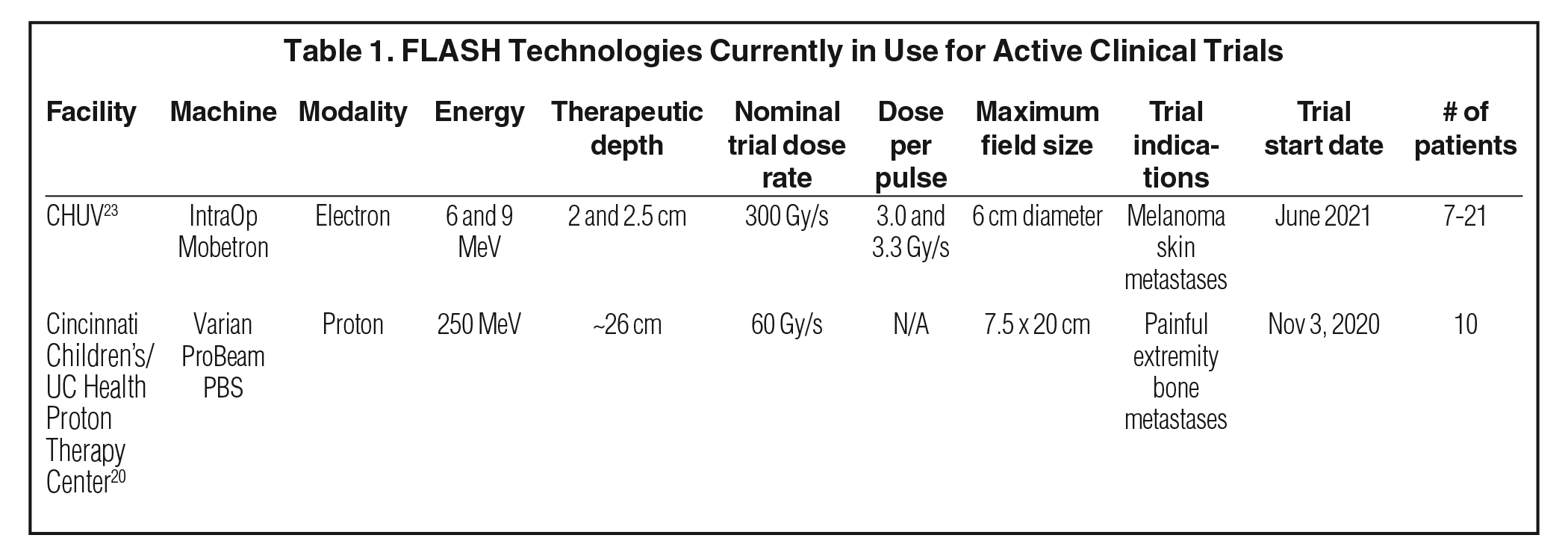
Two clinical trials are active at the time of writing of this review article. They each employ different radiation modalities and delivery methods, which are summarized in Table 1. The technologically feasible treatment parameters are discussed below.
Cincinnati Children’s/University of Cincinnati Health Proton Therapy Center (FAST-01)
Cincinnati Children’s/University of Cincinnati Health Proton Therapy Center is actively enrolling in a single-arm, prospective, feasibility trial named FAST-01 sponsored by Varian Medical Systems to treat painful extremity bone metastases. The trial started in November 2020 with the plan to enroll 10 patients with up to 3 painful extremity bone metastases without prior radiation therapy or other local therapy to the treatment sites.20 The goal of the trial is to assess the technical feasibility and safety of 8 Gy in 1 fraction of proton UHDR-RT for human treatment, and to evaluate the pain response and toxicity associated with this treatment.
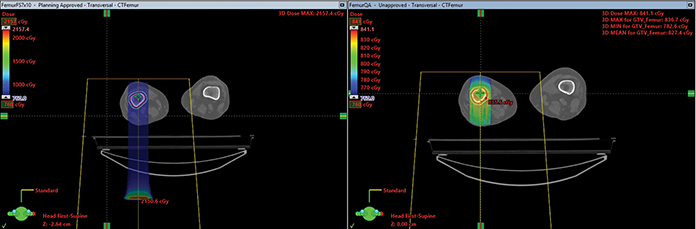
This trial utilizes a Varian ProBeam pencil-beam scanning gantry with no significant modification of the beam line or accelerator. The primary modifications are a primary dose monitor, rated for UHDR, and a change to the treatment planning workflow. The proton therapy system delivers a monoenergetic 250 MeV single-layer transmission radiation field at no less than 40 Gy/s and a nominal isocenter dose rate of 60 Gy/s. Transmission fields enter and exit through the patient’s body, thereby delivering therapeutic dose using the entrance plateau region of a Bragg peak, as opposed to using the Bragg peak region itself as in conventional proton therapy treatments (Figure 1). In some regards, the FAST-01 treatment plans are comparable to opposed-beam photon plans rather than intensity-modulated proton therapy or compensator-based passive scattering proton therapy.
Field sizes range from 7.5 × 7.5 cm2 to 7.5 × 20.0 cm2, which are suitable for treatment of a wide range of extremity tumors. The length of the plateau region of the beam is relatively homogenous up until the point where the Bragg peak begins to form, which is at a water-equivalent depth of 26 cm, and this point is defined as the maximum depth of treatment. However, a limitation of transmission fields is the lack of normal tissue sparing that would typically be achieved by elimination of exit dose from conventional Bragg peak fields.
Additional details regarding this system are published in Cunningham et al’s recent study on soft tissue and skin toxicity in mice.21 They describe delivering 35 Gy and 15 Gy to a 25 × 23 mm2 field at isocenter via single-layer spot patterns made up of 30 separate spots with a uniformity specification of ± 2.5%. The frequency of beam directly from the cyclotron is quasi-continuous, at approximately 72 MHz, and the spot patterns contain spots of equal weight and are scanned continuously. For this study, a maximum mean dose rate of 115.1 Gy/s was achievable at isocenter.
This configuration with its relatively wide range of field sizes and depths will allow for a plethora of clinical indications. The FAST-01 trial is an important clinical and technological starting point for proton pencil-beam scanning UHDR-RT and will pave the way for additional trials and technological developments in the future. The design of the FAST-02 trial for another palliative indication is already under way.22
Centre Hospitalier Universitaire Vaudois (CHUV) / Lausanne University Hospital
Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne University Hospital, performed the first human treatment using FLASH-RT.19 The team is now opening a FLASH-RT clinical trial that is enrolling as of June 2021. The approved phase I trial will determine the FLASH-RT dose that is able to provide durable tumor control for melanoma skin metastases without causing significant toxicity, with a goal to enroll 7 to 21 patients (Figure 2).
This trial utilizes an IntraOp Mobetron mobile linac optimized for UHDR delivery,23 conventionally used intraoperatively or for dermatologic treatments. To accomplish UHDR, the control system was modified to enable prescribing the number of pulses for delivery, setting the number of pulses for both the electron gun and solid-state modulator.23 The pulse width and pulse frequency are programmable and can be set from 0.5-4 μs and 5-90 Hz, respectively.
The Mobetron unit was commissioned for 6 and 9 MeV nominal energies using conventional protocols for commissioning of a medical linac as per the guidelines of AAPM TG-72.24 As per Moeckli et al, commissioning was performed at the linac exit window, corresponding to a source-to-surface distance (SSD) of 17.3 cm, representing the maximal mean dose rate that can be achieved, as well as 20 cm further at 37.3 cm SSD, to be used for treatment under clinical protocol. This SSD corresponded to mean dose rates of ~300 Gy/s, similar to their preclinical experiments as well as for the first patient treated with FLASH-RT.19
At the protocol-specified treatment SSD of 37.3 cm, maximum dose-per-pulses of 3 Gy and 3.3 Gy is achieved for 6 and 9 MeV energies, respectively, with treatment depths – defined as the depth beyond the depth dose maximum at which 90% of the maximum dose is seen (R90) – of 2 to 2.5 cm, well-suited for cutaneous treatments. Treatment field sizes, defined by the 90% isodose line, are at a 6 cm maximum at treatment SSD.
Available UHDR-RT Technologies Enabling Future Clinical Trials
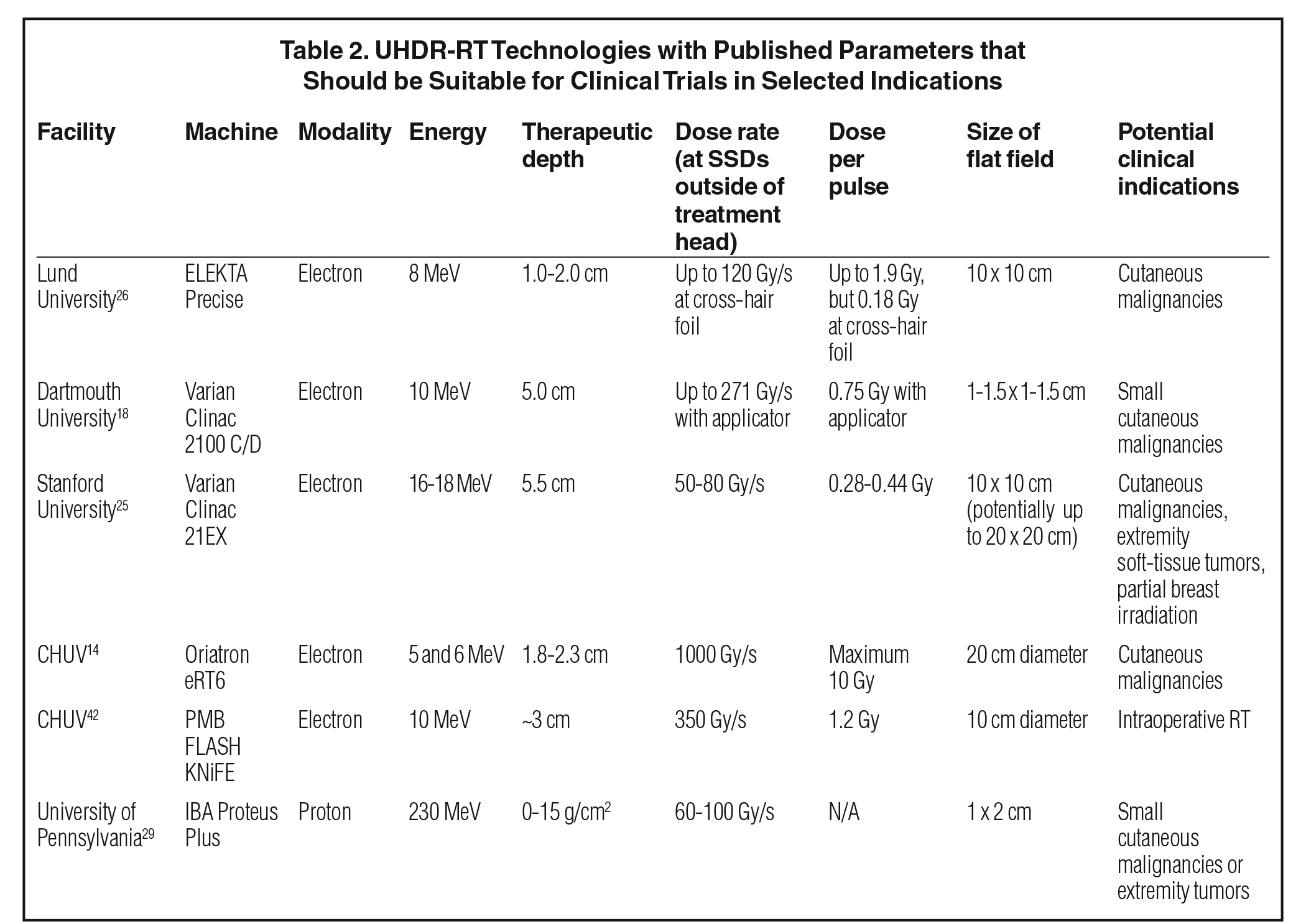
Current technologies for UHDR-RT delivery that have potential for future clinical trial use are summarized in Table 2.
Dedicated Electron UHDR Treatment Machines
Several dedicated electron UHDR-RT systems have been developed. The Oriatron eRT6 has been used for the first human treatment, as discussed previously.19 This system was custom built by PMB ALCEN and commissioned by CHUV to deliver electrons with 5-6 MeV energy with a maximum dose-per-pulse of 10 Gy with a pulse repetition frequency of 5-200 Hz. The maximum average dose rate is 1000 Gy/s. The eRT6 is capable of delivering UHDR at a conventional treatment SSD of 100 cm with field sizes from 1.6 to 20 cm with an R80 of 1.8 to 2.3 cm.14
The IntraOp Mobetron intraoperative RT system is another dedicated electron UHDR machine that is discussed in the CHUV section above. In addition to CHUV, this UHDR system exists at Ohio State University Comprehensive Cancer Center, The University of Texas MD Anderson Cancer Center, University of California Irvine, and the Centre Hospitalier de l’Université de Montreal. In 2021, a commissioning paper was published with details of the system.23
Given the R80 value of the eRT6 (1.8 to 2.3 cm) and the R90 values of the Mobetron system (2 to 2.5 cm), these systems are most suitable for clinical trials involving cutaneous lesions, as well as intraoperative RT.
Clinical Linac-Based Electron UHDR-RT Delivery
To increase accessibility to FLASH- RT utilization and research, multiple groups have developed configurations using clinical linacs to output electron UHDR-RT. Without the need for a dedicated specialized UHDR machine, there is potential for a wider range of radiation teams and centers to be able to conduct future clinical trials.
Schüler et al at Stanford University configured a Varian Clinac 21EX for small animal irradiation.17 They tuned the beam using a custom 20 MeV program printed circuit board to customize the control parameters, with the gun current and radiofrequency driver manually adjusted to achieve the maximum dose rate. The measured output showed a percentage depth dose (PDD) curve similar to that of 16-18 MeV conventional electron output; 220 Gy/s was attainable at the level of the mirror, which was used for animal experiments, with a field diameter encompassed by the 90% isodose level of 4.1 cm. Additional work is being conducted by No and Wu et al using a novel configuration on a Varian Trilogy that uses a flat electron-arc applicator in place of a standard electron cone, with the scattering foil retained in the beam’s path (Figure 3).25 This has shown UHDRs of 50 to 80 Gy/s at SSDs of 90 and 70 cm, respectively. The output is a flat, symmetrical beam with an 80% dose diameter of at least 10 cm (potentially up to 20 cm), with an R90 of 5.5 cm. This configuration is limited by the dose rate decreasing due to the presence of the scattering foil, which limits the SSDs that can be used to maintain UHDRs. However, the relatively large range of treatment field sizes will allow for potential future clinical trials on superficial tumors, such as cutaneous malignancies, sarcomas, or partial breast irradiation.
Lempart et al at Skåne University Hospital and Lund University in Sweden have modified an Elekta Precise clinical linac to deliver electron UHDR-RT.26 The team manually adjusted the gun current, modulator charge rate, and beam steering values, as well as disabled the interlocks to operate the machine in electron mode without the electron applicator. With the scattering foils in the beam’s path, dose rates of 30 and 300 Gy/s were achieved at the cross-hair foil (53 cm SSD) and at the wedge position (19 cm SSD), respectively, with the beams resembling 8 MeV electrons. Beam flatness of < 5% was found for a 20 × 20 cm2 area and for a 2 cm diameter circular area, respectively, at those positions. When the scattering foils were removed, the dose rates increased to 120 to 1000 Gy/s, respectively, and the areas of beam flatness < 5% were reduced to 10 x 10 cm2 and a 1.5 cm diameter circle, respectively. As such, at the clinically practical SSD position (ie, outside of the gantry head) published in this study of 53 cm SSD, the scattering foils had to be removed to achieve UHDRs, which limited the flat beam width to 10 cm. Furthermore, they observed that the total dose delivered seemed to become unstable (standard deviation increased to 7% to 11%) when >10 minutes passed after the machine warm-up procedure, although this improved with fine-tuning of the resonance frequency of the accelerator. As part of unpublished work, the team has been able to configure this system to produce a beam at a dose rate of 200 Gy/s at 100 cm SSD with a flat field size of 12 12 cm2 (personal communication).
Rahman and Ashraf et al at Dartmouth University have developed a configuration on a Varian Clinac 2100 C/D whereby the team removed the x-ray target, flattening filter, and scattering foil from the path of the beam and selected a 10 MV photon beam energy.18 With this set-up, a dose rate of 310 Gy/s at 100 cm SSD and depth of 4 cm with the jaws wide open (40 40 cm2 field size) were achievable. Using an electron applicator, they found dose rates of 271 Gy/s with a 2 cm circular cutout and 235 Gy/s with a 1 cm circular cutout. The practical range of depth was approximately 5 cm. However, the team found that the dose per pulse required a “ramp-up” and did not become stable until delivery of ~10 pulses. Also, the beam profile was Gaussian in the absence of the flattening filter and scattering foil, which made for a relatively narrow flat beam width. Experiments on animal tumor models and clinical veterinary treatments are underway using this configuration. There is potential to treat patients in the future, with a possible upcoming feasibility trial on treating patients with advanced skin lesions that are surgically unresectable.27
Proton UHDR-RT
Currently, the technology for proton UHDR-RT has shown dose rates ≥ 40 Gy/s with proton pencil beams, but challenges exist with attaining mean dose rates in the UHDR range in a larger volume with a spread-out Bragg peak.28 As such, many proton UHDR systems utilize a transmission radiation field, which directs the plateau region of the beam through the entire thickness of the body such that the proton beam enters, exits, and then stops outside the body.21,28 The “FLASHForward Consortium” sponsored by Varian is an aggregate of 20 institutions (and growing) in the US, Europe, and Asia, representing radiation therapy centers with research programs in FLASH-RT with the goal of advancing research and clinical applicability of proton UHDR-RT.22
The Varian ProBeam system has been used for proton UHDR-RT and has been discussed above in the section on the FAST-01 clinical trial.
The IBA Proteus Plus system, another clinical proton machine, can deliver proton UHDR-RT with energies up to 230 MeV. Diffenderfer et al at the University of Pennsylvania created a configuration of this system whereby a double-scattered proton beam was delivered quasi-continuously at 106 MHz with a beam current up to 300 nA.29 They were able to achieve mean dose rates of 60 to 100 Gy/s at isocenter. Homogenous dosimetry was observed within a range of 0 to 15 g/cm2. This configuration has been used for mice experiments with a collimated beam size of 1 × 2 cm2. This same group conducted a simulation experiment where they theorized that a beam current of > 500 nA would provide an effective field dose rate of ≥ 40 Gy/s for a field size of 4 × 4 cm2. However, this did not account for scanning magnet slew time and energy switching time, which the authors discussed were limiting factors in achieving larger field sizes. The IBA Proteus Plus system was also used by Beyreuther et al for experiments on zebrafish embryos, where 100 Gy/s was delivered to a 6.5 mm diameter area.30
There are potential solutions to increase the field size of proton UHDR-RT while still reaping the benefits of the Bragg peak. For instance, passive scattering and the use of ridge filters can produce larger fields, but this leads to particle loss and decreased dose rate, as well as requiring significantly higher incident beam currents. Pencil-beam scanning is another possible option that can produce UHDRs at individual spots, but maintaining the dose rates across the entire treatment volume can be limited by the speed of the scanning magnets and the penumbra between scanning layers. Indeed, further experiments are required to assess the feasibility of these and other configurations for proton UHDR-RT, as well as their consequent biological effects.29,31
Upcoming FLASH-RT Technologies
Pluridirectional High-energy Agile Scanning Electronic Radiotherapy (PHASER)
Current state-of-the-art clinical RT machines based on x-rays can deliver highly conformal doses with image guidance to general large-volume deep-seated cancer targets, but are orders of magnitude too slow to deliver UHDR-RT owing in large part to the inefficiency of bremsstrahlung x-ray production and inherently slow mechanical systems for gantry rotation and intensity modulation. Major technical hurdles must therefore be overcome to deliver conformal photon FLASH-RT. Researchers at Stanford and the SLAC National Accelerator Laboratory discovered novel particle accelerator principles, originally conceived to overcome breakdown in ultrahigh gradient (>100 MeV/m) accelerator structures, which also greatly increase the radio-frequency (RF) power efficiency. This, combined with novel strategies to eliminate slow mechanical components, forms the basis of pluridirectional high-energy agile scanning electronic radiotherapy (PHASER).32
In the distributed RF-coupling architecture with genetically optimized cell design (DRAGON) for electron accelerators, the shape of the accelerating cells is optimized to minimize the peak surface magnetic fields, a key contributor to RF power loss to generating waste heat in the accelerator structure. More efficient transfer of RF power to the electron beam and the ability of the accelerator to operate with a higher duty factor without exceeding temperature limits combine to enable 30-fold higher beam current compared with conventional clinical linacs operating at 10 MV energy.33 A phased-array RF power network allows combining the output power of multiple small, lower voltage RF power sources (klystrinos) and rapidly switching the summed power to any one of an array of linac beamlines (eg, 16) arranged around the patient to provide irradiation from multiple angles in rapid succession for conformal RT, eliminating the need for a mechanical rotating gantry and providing a compact overall form factor. Intensity-modulation from each direction can also be achieved electronically by scanning the electron beam in conjunction with an extended bremsstrahlung conversion target and multichannel collimator array to produce scanning x-ray beamlets. Figure 4 illustrates the core components that make up PHASER.
Very High-energy Electrons (VHEE)
As electron energy increases from conventional 4-20 MeV to very high-energy (eg, > 100 MeV), the depth-dose characteristics of the beam change from only superficially penetrating to deep penetration with lower entrance and exit dose for a given dose at depth compared to MV energy x-rays.34-36 As a possible short-term path to clinical FLASH applications, the Stanford group simulated the impact of applying higher peak RF power (through pulse compression of output from a commercial klystron) to the 10 MeV DRAGON linac designed for the PHASER platform, finding that 40 MeV acceleration would be achievable at a beam current sufficient for UHDR when treating directly with electrons.37 Opposing beams at this energy could produce a homogeneous dose distribution similar to a photon plan for anatomic sites with modest thickness, such as a pediatric brain, at dose rates up to > 400 Gy/s. The same principles are being used to design compact high-gradient accelerators with 100+ MeV beam energy for very high-energy electron (VHEE)-based conformal FLASH therapy.32
Another technology capable of delivering FLASH dose rates with VHEE is being investigated by researchers at CHUV and CERN.38 The full details of this technology are not yet available at the time of this writing but report a conceptual design of a unique apparatus based on a compact linear collider (CLIC) accelerator technology enabled to accelerate electrons to treat tumors up to 15 to 20 cm in depth. We anticipate the proposed technology, noted to be capable of treating large and deep-seated tumors, would likely offer high clinical relevance.
Conclusion/Discussion
FLASH-RT holds exciting promise as technological advancements occur at a rapid pace. Preclinical studies show great potential for FLASH-RT to widen the therapeutic ratio in radiation treatments. Novel upcoming clinical trials, enabled by the development of new technologies in RT, are helping to test those preclinical findings for human translation.
As future trials in FLASH-RT develop, several considerations arise from limitations of current technologies. Electron therapy has limited tissue penetration, and is suitable mainly for superficial tumors and intraoperative RT.39 However, this limitation could potentially be remedied with the use of VHEE, which has the penetration required for deep-seated tumors. Current linacs for conventional photon delivery cannot reach UHDRs. Solutions for this require novel innovations in linac development, such as those in the PHASER system.32 While proton beam therapy is highly suited for targeting deep-seated tumors with normal tissue-sparing dosimetry, currently UHDR is achieved using transmission fields from a single-beam direction, which forfeits the conformity advantage of protons. FLASH treatments taking advantage of the Bragg peak are under development for more conformal treatment. Electron and proton-based FLASH platforms have already entered clinical trials for select indications, and Bragg peak proton FLASH delivery will likely be implemented clinically in the next 1-2 years. Meanwhile, x-ray and VHEE FLASH are actively under development and may reach the clinic within 3-5 years. Within this timeframe, however, much more research is needed for the clinical implementation of FLASH. Currently, the pulse structure, repetition rates, and other beam characteristics that are required to obtain an optimal FLASH effect are yet unknown, as well as whether these requirements are both strictly necessary and sufficient. Given the short beam on time, new technologies for accurate dose monitoring will need to be developed, including QA and calibrations procedures. While some of these technologies are costly for adoption by most clinics, current superficial electron FLASH and developing x-ray FLASH technology have the potential to be economical compared to conventional medical linacs and compatible with existing clinical vaults.
It is also important to mention that there are challenges in comparing FLASH study results between different modalities, as they vary significantly in physical parameters such as pulse structure, time structure, and definition of dose rate. Additionally, while many studies focus on the mean dose rate as the primary driver of the FLASH effect, more complex factors are likely at play, inclusive of dose per pulse, the total number of pulses, and the dose-rate within the pulse.5 Also, as the biological mechanisms underlying the FLASH effect are still in question, the impact of different modalities on inducing this effect is an important topic of investigation.
As highlighted in this review, there is currently wide variability in UHDR-RT delivery spanning multiple modalities and delivery methods. Future standardization is essential in the development of larger UHDR-RT clinical trials that span different research teams and institutions. Initial steps to address this have begun,40 with exploration of methods to precisely measure the delivered UHDR radiation, which can then lead to reference standards and dosimetry methods. This would allow for both stringent quality assurance and comparison across different RT modalities, configurations, and experimental settings.41
As preclinical data on FLASH-RT expands, and radiation therapy technology continues to advance, the converging of the two have heralded the beginning of FLASH human clinical trials. Many complex questions remain, including optimal indications, whether the FLASH effect translates from animal models to patients, the selection of treatment modality, and the implementation of dosimetry / quality assurance. By being vigilant in this next step into clinical translation of this new technology, we can carefully unlock the vast potential impact that FLASH-RT may have on radiation treatment and oncologic care at large.
References
- Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6(245):245ra93. doi:10.1126/scitranslmed.3008973
- Levy K, Natarajan S, Wang J, et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci Rep. 2020;10(1):21600. doi:10.1038/s41598-020-78017-7
- Wilson JD, Hammond EM, Higgins GS, Petersson K. Ultra-high dose rate (FLASH) radiotherapy: silver bullet or fool’s gold? Front Oncol. 2019;9:1563. doi:10.3389/fonc.2019.01563
- Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother Oncol. 2017;124(3):365-369. doi:10.1016/j.radonc.2017.05.003
- Bourhis J, Montay-Gruel P, Gonçalves Jorge P, et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother Oncol. 2019;139:11-17. doi:10.1016/j.radonc.2019.04.008
- Hornsey S, Alper T. Unexpected dose-rate effect in the killing of mice by radiation. Nature. 1966;210(5032):212-213. doi:10.1038/210212a0
- Field SB, Bewley DK. Effects of dose-rate on the radiation response of rat skin. Int J Radiat Biol Relat Stud Phys Chem Med. 1974;26(3):259-267. doi:10.1080/09553007414551221
- Berry RJ, Hall EJ, Forster DW, Storr TH, Goodman MJ. Survival of mammalian cells exposed to x rays at ultra-high dose-rates. Br J Radiol. 1969;42(494):102-107. doi:10.1259/0007-1285-42-494-102
- Simmons DA, Lartey FM, Schüler E, et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol. 2019;139:4-10. doi:10.1016/j.radonc.2019.06.006
- Alaghband Y, Cheeks SN, Allen BD, et al. Neuroprotection of radiosensitive juvenile mice by ultra-high dose rate FLASH irradiation. Cancers (Basel). 2020;12(6). doi:10.3390/cancers12061671
- Huang CC, Mendonca MS. News FLASH-RT: to treat GBM and spare cognition, fraction size and total dose matter. Clin Cancer Res. 2021;27(3):662-664. doi:10.1158/1078-0432.CCR-20-4067
- Soto LA, Casey KM, Wang J, et al. FLASH irradiation results in reduced severe skin toxicity compared to conventional-dose-rate irradiation. Radiat Res. 2020;194(6):618-624. doi:10.1667/RADE-20-00090
- Vozenin M-C, Hendry JH, Limoli CL. Biological benefits of ultra-high dose rate FLASH radiotherapy: sleeping beauty awoken. Clin Oncol (R Coll Radiol). 2019;31(7):407-415. doi:10.1016/j.clon.2019.04.001
- Jaccard M, Durán MT, Petersson K, et al. High dose-per-pulse electron beam dosimetry: commissioning of the Oriatron eRT6 prototype linear accelerator for preclinical use. Med Phys. 2018;45(2):863-874. doi:10.1002/mp.12713
- Patriarca A, Fouillade C, Auger M, et al. Experimental set-up for FLASH proton irradiation of small animals using a clinical system. Int J Radiat Oncol Biol Phys. 2018;102(3):619-626. doi:10.1016/j.ijrobp.2018.06.403
- Montay-Gruel P, Bouchet A, Jaccard M, et al. X-rays can trigger the FLASH effect: ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol. 2018;129(3):582-588. doi:10.1016/j.radonc.2018.08.016
- Schüler E, Trovati S, King G, et al. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int J Radiat Oncol Biol Phys. 2017;97(1):195-203. doi:10.1016/j.ijrobp.2016.09.018
- Rahman M, Ashraf MR, Zhang R, et al. Electron FLASH delivery at treatment room isocenter for efficient reversible conversion of a clinical LINAC. Int J Radiat Oncol Biol Phys. Published online January 2021. doi:10.1016/j.ijrobp.2021.01.011
- Bourhis J, Sozzi WJ, Jorge PG, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18-22. doi:10.1016/j.radonc.2019.06.019
- Breneman J. Feasibility study of FLASH radiotherapy for the treatment of symptomatic bone metastases (FAST-01). Accessed May 17, 2021. https://www.clinicaltrials.gov/ct2/show/NCT04592887
- Cunningham S, McCauley S, Vairamani K, et al. FLASH proton pencil beam scanning irradiation minimizes radiation-induced leg contracture and skin toxicity in mice. Cancers (Basel). 2021;13(5). doi:10.3390/cancers13051012
- FlashForward Consortium. Accessed November 5, 2021. https://www.varian.com/about-varian/research/flashforward-consortium
- Moeckli R, Gonçalves Jorge P, Grilj V, et al. Commissioning of an ultra-high dose rate pulsed electron beam medical LINAC for FLASH RT pre-clinical animal experiments and future clinical human protocols. Med Phys. 2021;48:3134-3142. doi:10.1002/mp.14885
- Beddar AS, Biggs PJ, Chang S, et al. Intraoperative radiation therapy using mobile electron linear accelerators: report of AAPM Radiation Therapy Committee Task Group No. 72. Med Phys. 2006;33(5):1476-1489. doi:10.1118/1.2194447
- No H, Wu Y, Manjappa R, et al. Feasibility of clinically practical ultra-high dose rate (FLASH) radiation delivery by a reversible configuration of a standard clinical-use linear accelerator. In: Accepted Oral Scientific Session; ASTRO Annual Meeting; 2021.
- Lempart M, Blad B, Adrian G, et al. Modifying a clinical linear accelerator for delivery of ultra-high dose rate irradiation. Radiother Oncol. 2019;139:40-45. doi:10.1016/j.radonc.2019.01.031
- Stahler L. Dartmouth researchers Pilot FLASH radiotherapy beam development for treatment of cancer. Dartmouth Geisel School of Medicine news. Published 2021. Accessed November 5, 2021. https://geiselmed.dartmouth.edu/news/2021/dartmouth-researchers-pilot-flash-radiotherapy-beam-development-for-treatment-of-cancer/
- Esplen N, Mendonca MS, Bazalova-Carter M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: a topical review. Phys Med Biol. 2020;65(23):23TR03. doi:10.1088/1361-6560/abaa28
- Diffenderfer ES, Verginadis II, Kim MM, et al. Design, implementation, and in vivo validation of a novel proton flash radiation therapy system. Int J Radiat Oncol Biol Phys. 2020;106(2):440-448. doi:10.1016/j.ijrobp.2019.10.049
- Beyreuther E, Brand M, Hans S, et al. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother Oncol. 2019;139:46-50. doi:10.1016/j.radonc.2019.06.024
- Nesteruk KP, Psoroulas S. FLASH irradiation with proton beams: beam characteristics and their implications for beam diagnostics. Appl Sci. 2021;11(5). doi:10.3390/app11052170
- Maxim PG, Tantawi SG, Loo BWJ. PHASER: a platform for clinical translation of FLASH cancer radiotherapy. Radiother Oncol. 2019;139:28-33. doi:10.1016/j.radonc.2019.05.005.
- Tantawi S, Nasr M, Li Z, Limborg C, Borchard P. Distributed coupling accelerator structures: a new paradigm for high gradient linacs. arXiv Prepr arXiv181109925. Published online 2018.
- Bazalova-Carter M, Liu M, Palma B, et al. Comparison of film measurements and Monte Carlo simulations of dose delivered with very high-energy electron beams in a polystyrene phantom. Med Phys. 2015;42(4):1606-1613. doi:10.1118/1.4914371
- Schüler E, Eriksson K, Hynning E, et al. Very high-energy electron (VHEE) beams in radiation therapy; treatment plan comparison between VHEE, VMAT, and PPBS. Med Phys. 2017;44(6):2544-2555. doi:10.1002/mp.12233
- DesRosiers C, Moskvin V, Bielajew AF, Papiez L. 150-250 meV electron beams in radiation therapy. Phys Med Biol. 2000;45(7):1781-1805. doi:10.1088/0031-9155/45/7/306
- Breitkreutz DY, Shumail M, Bush KK, Tantawi SG, Maxime PG, Loo BW. Initial steps towards a clinical FLASH radiotherapy system: pediatric whole brain irradiation with 40 MeV electrons at FLASH Dose Rates. Radiat Res. 2020;194(6):594-599. doi:10.1667/RADE-20-00069.1
- CERN. CERN and Lausanne University Hospital collaborate on a pioneering new cancer radiotherapy facility. Published 2020. Accessed October 6, 2021. https://home.cern/news/news/knowledge-sharing/cern-and-lausanne-university-hospital-collaborate-pioneering-new-cancer
- Montay-Gruel P, Meziani L, Yakkala C, Vozenin M-C. Expanding the therapeutic index of radiation therapy by normal tissue protection. Br J Radiol. 2019;92(1093):20180008. doi:10.1259/bjr.20180008
- Schüller A, Heinrich S, Fouillade C, et al. The European Joint Research Project UHDpulse - metrology for advanced radiotherapy using particle beams with ultra-high pulse dose rates. Phys Med. 2020;80:134-150. doi:10.1016/j.ejmp.2020.09.020
- Velalopoulou A, Koumenis C. FLASH radiotherapy: Are we ready for clinical translation? Spring 2021 ASTRONews. Published online 2021;(24)1.
- PMB-Alcen. FLASHKNiFE: the FLASH radiotherapy system. Accessed June 2, 2021. https://www.pmb-alcen.com/en/flashknife-flash-radiotherapy-system
Citation
Y W, HJ N, DY M, AE M, R M, J B, E S, PG M, Jr LB. Technological Basis for Clinical Trials in FLASH Radiation Therapy: A Review. Appl Radiat Oncol. 2021;(2):6-14.
July 27, 2021