Stereotactic ablative radiation therapy in the treatment of liver tumors
Images
SA-CME credits are available for this article here.
Liver cancer-related death rates continue to accelerate worldwide.1,2 Numerous local techniques are evolving to address the growing burden of disease. These techniques include surgery (partial liver resection or liver transplant), ablation (radiofrequency, microwave, ethanol, cryoablation), and intra-arterial injections (chemoembolization, radioembolization, bland embolization). Systemic treatments, such as sorafenib, regorafenib, or nivolumab, are also expanding. An additional option is stereotactic ablative radiation therapy (SABR). SABR has harnessed innovations in external-beam radiation therapy delivery and toxicity modeling to safely and noninvasively deliver high radiation therapy doses to liver tumors in only 1 to 5 treatments. Here we review the indications, efficacy, toxicity and methods for SABR in liver tumors. While prospective comparative data is lacking between SABR and other local techniques, we suggest that SABR offers high local control, low toxicity, and ability to treat a range of tumor volumes and locations in a precise, noninvasive manner. While choice of local liver tumor therapy is currently institution-specific, future utilization of liver SABR promises to increase with experience and recognition.
SABR in the Treatment of Primary Liver Cancer
Hepatocellular Carcinoma (HCC)
HCC is the most common primary liver cancer in the world, with a four-fold increase in incidence over the last 40 years in the United States.3 Partial liver resection or orthotopic liver transplant (OLT) remain the accepted first-line treatments for eligible patients.4,5
Patients waiting for OLT are at risk for disease progression. Clinical series demonstrate that SABR can prevent HCC progression prior to transplant. Sapisochin et al compared SABR (n = 36) with transcatheter arterial chemoembolization (TACE) (n = 99) and radiofrequency ablation (RFA) (n = 244) as bridges to OLT. They found that drop-out rate, post-transplant survival and HCC recurrence were similar for all techniques, despite SABR treating a greater tumor burden than RFA: an average of 2 lesions to 1, 4.5 cm diameter to 2.5 cm, and a higher mean Model of End-stage Liver Disease (MELD) score.6
In the United States, 70% to 90% of all HCC cases occur with cirrhosis, and many patients are unsuitable for resection.7 For patients unable to undergo definitive resection, Table 1 summarizes studies demonstrating that SABR is an excellent option for tumor control with limited toxicity. No randomized data exist to prove superiority of SABR compared to other techniques. Nevertheless, a 2016 retrospective study from the University of Michigan compared SABR (n = 63 treated with 27 to 60 Gy in 3 to 5 fractions) to RFA (n = 161), showing they are equally effective for treating inoperable HCC < 2 cm, but that SABR provides better local control (LC) than RFA for lesions ≥ 2 cm.8 A second retrospective investigation compared SABR and TACE, with 2-year LC significantly better for SABR, 91.3% to 22.9%, respectively, and no significant difference in overall survival (OS).9
An advantage of SABR compared with other local techniques is that lesions can be treated that are difficult to access by RFA, embolization or surgery (eg, large volume tumors; disease complicated by portal thrombus;10 and lesions near the liver capsule, major vessels or diaphragm).
Intrahepatic Cholangiocarcinoma
Intrahepatic cholangiocarcinoma (ICC) is the second-most common primary liver cancer worldwide, representing 10% to 20% of liver cancer diagnoses. Surgery is the only curative treatment for local disease, but up to 70% of ICC is unresectable.11
In 2016, physicians from MD Anderson Cancer Center and Harvard analyzed outcomes from a series of unresectable ICC patients who received chemotherapy followed by moderately hypofractionated radiation therapy and identified a survival advantage with dose escalation.12 Patients treated to a biologically equivalent dose (BED) > 80.5 Gy had almost double the 3-year survival of those treated to lower doses (73% to 38%, respectively).
Princess Margaret Hospital conducted the first phase I trial using SABR to treat inoperable ICC. Ten patients were treated to a median dose of 36 Gy in 6 fractions, with 1-year LC of 65% and median OS of 15 months, an improvement over historic controls. There were no cases of radiation-induced liver disease (RILD), and toxicities were grade 3 or less.13
SABR in the Teatment of Liver Metastases
Each year, 30 000 patients with colorectal cancer (CRC) are found to have oligometastatic disease (OMD) limited to the liver either on presentation or at recurrence.2,14,15 In 2016, the European Society for Medical Oncology recommended the use of SABR in combination with systemic agents to treat unresectable colorectal OMD.16 In the last decade, several phase I and II studies using SABR to treat hepatic OMD from favorable primaries have reported 2-year LC rates > 90%, and median OS significantly higher than historical controls treated with systemic therapy alone (29 to 32 months vs. 24 months for chemotherapy).17-19 In a large retrospective series studying outcomes from SABR treatment of mainly hepatic OMD, Fode et al identified 5 factors associated with favorable survival: World Health Organization (WHO) performance status 0-1, solitary metastasis, size ≤ 3 cm, metachronous metastases and pre-SBRT systemic therapy. BED10 > 100 Gy correlated with low local recurrence rates.20
The recent CLOCC trial (chemotherapy + local ablation vs chemotherapy) randomized 119 patients with liver-only colorectal unresectable metastatic disease to systemic therapy alone vs systemic therapy with RFA and surgical resection (when possible), and demonstrated a median OS benefit with local therapy (45.6 months vs 40.5 months).21 While no local ablative technique has demonstrated superiority compared to another local ablative technique in a randomized trial, Stintzing et al performed a matched comparative analysis of 60 patients with unresectable colorectal liver metastases, divided between SABR (24 to 26 Gy in 1 fraction) and RFA. One-year LC favored SABR (85%) compared to RFA (65%).22 This suggests that SABR could further enhance survival benefits for unresectable liver metastases compared to RFA.
SABR also provides excellent control of oligometastatic liver disease from noncolorectal primaries. A 2016 series demonstrated 100% 2-year LC rates for 58 noncolorectal liver metastases.23 Additional studies of SABR for liver metastases are summarized in Table 2.
SABR Technique
Dose
In practice, the authors of this manuscript generally follow the isotoxicity approach initially proposed by Dawson et al and adapted into the Radiation Therapy Oncology Group (RTOG) 1112 trial (NCT01730937) protocol.24,25. In RTOG 1112, 5-fraction SABR is prescribed to Child-Turcotte-Pugh class (CTP) A HCC, and the mean liver dose (MLD, defined as liver minus gross tumor volume [GTV]) determines the prescription dose based on an expected 5% incidence of RILD. If the MLD in the achieved plan is less than 13 Gy, the dose is 50 Gy over 5 fractions; however, the dose is reduced as MLD increases. Caution must be employed for dose to adjacent stomach and bowel, and additional dose constraints are also provided within the RTOG 1112 protocol.
Logically, this schema can also be applied to CTP A patients with liver metastases or cholangiocarcinoma, as BED10 = 100 Gy (50 Gy in 5 fractions) correlates with good tumor control.12,20 For patients with limited liver metastatic disease without underlying liver dysfunction, 60 Gy in 5 fractions (BED10 = 132 Gy) can be considered. Conversely, caution must be used in CTP B patients. A phase I/II trial reported 38% grade 3 or higher toxicities for CTP B HCC patients treated with SABR.26 The use of dose escalation in this fragile population requires careful patient selection. For CTP C patients, hospice should be considered.
Image Guidance and Respiratory Management
Since increasing MLD correlates with increasing rates of RILD and limits prescription dose and anticipated tumor control, attempts should be made to reduce the MLD.27,28 Custom immobilization, image guidance and respiratory management allow reduction of the planning target volume (PTV) margin to about 5 mm.
Patients with limited respiratory motion assessed by fluoroscopy, 4-dimensional computed tomography (4D-CT), or cine magnetic resonance imaging (MRI) could be treated with an internal target volume (ITV) encompassing the respiratory excursion plus PTV expansion for setup uncertainty. However, craniocaudal and anterior-posterior excursions of liver tumors of 2 to 3 cm have been reported with limited motion reduction by abdominal compression.29 Therefore, appropriate motion management techniques must be available to treat patients with large respiratory motions. Example strategies include respiratory gating, breath-hold and active tracking.30-32 Such systems include the Cyberknife Synchrony (Accuray, Sunnyvale, California) and Varian Real-Time Position Management (Varian, Palo Alto, California) systems, which use cameras during therapy to track markers placed on the body’s surface that are correlated to the internal tumor position. A common alternative is Elekta’s Active Breathing Coordinator (Elekta, Stockholm, Sweden), which tracks and assists reproducible lung filling during treatment.
These systems require internal calibration of the target position to the tracking system using fluoroscopy or breath-hold cone-beam CT at the beginning of each treatment. For these x-ray-based image guidance techniques, we strongly recommend target localization with radiopaque fiducials placed prior to simulation.33 This has been shown to lower the maximum setup error from 12 mm (based on diaphragm position and bony landmarks) to 2 mm.34 Residual Lipiodal (Guerbet, Villepinte, France) injected from prior TACE treatments can also be used.35
Definition of the target requires intravenous (IV) contrast at the time of simulation and/or careful fusion to diagnostic scans. If gating or breath-hold is employed, simulation must include images for planning in that respiratory phase.
Emerging Techniques
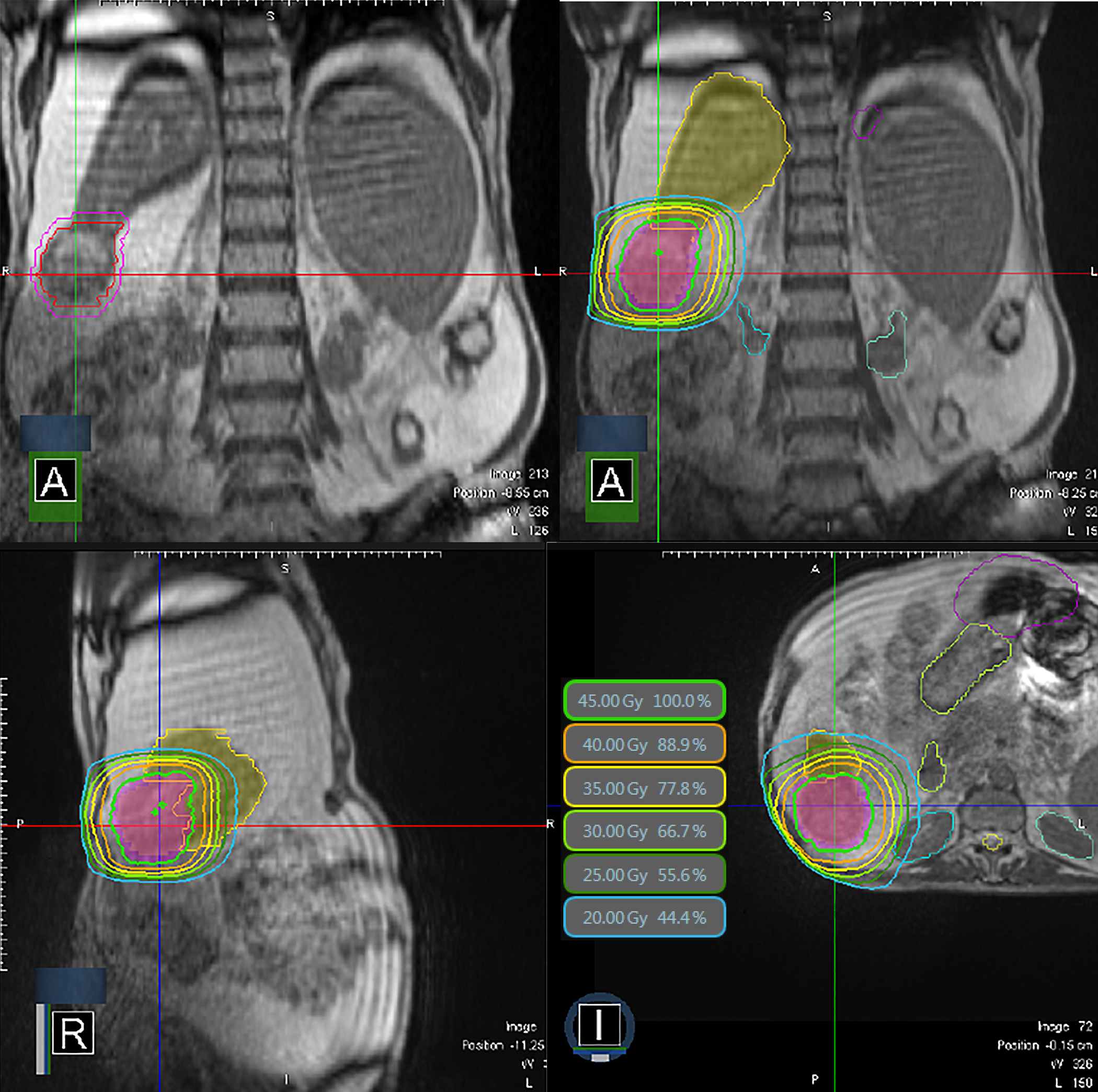
Recently, an MRI-guided radiation therapy system has become available for treatment of liver tumors.36,37 MRI simplifies the SABR procedure since it enables direct tumor visualization for planning and daily setup as well as near real-time imaging during treatment. An example of MRI-guided treatment is shown in Figure 1. Real-time visualization of liver targets can be further enhanced by use of gadoxetate MRI contrast.38
In some settings, proton SABR could enhance normal liver sparing compared to conventional photon treatments given the reduced exit dose from the Bragg peak, reducing MLD and increasing the size or dose of treatment.39,40 Nevertheless, respiratory motion offers more complications in proton than photon treatment due to derangement of the Bragg peak location caused by target depth variability. Use of proton SABR has been limited historically because few proton therapy centers were equipped with respiratory gating; however, the number of capable centers is increasing.41,42
Radiation-induced Liver Disease
Recognition of the liver’s parallel functional structure, reinforced by surgical experience and refined through advances in Normal Tissue Complication Probability (NTCP) modeling, provides the physiologic justification for partial-liver irradiation.43 When one-third of the normal liver parenchyma (standardized to 700 cc of tissue) is protected from doses > 15 Gy in 3-5 fractions, the risk of RILD is < 5% for patients with baseline CTP A hepatic function.44
RILD is the most common dose-limiting toxicity for radiation therapy of liver tumors with time-to-onset ranging from 2 weeks to 8 months post-treatment. Classical RILD is characterized by fatigue, anicteric ascites, elevation of alkaline phosphatase out of proportion to other live enzymes, abdominal pain, and hepatomegaly. Nonclassical RILD patients present with jaundice and elevated serum transaminase. Given the overlap with liver failure of other causes, such as hepatitis, it is often difficult to directly ascribe to radiation therapy. Management is supportive, similar to management of other types of liver injury.
In practice, patients generally report transient loss of appetite and increased fatigue resolving by 3 months following SABR, with pretreatment quality of life maintained through 1 year.45
Conclusion
Numerous studies support SABR for the treatment of liver tumors such as unresectable hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and liver metastases. Careful consideration of image guidance and respiratory management allows for minimization of normal liver treated, improving the safety, effectiveness, and size and number of tumors that can be treated successfully. Comparative studies to other techniques, improving radiation therapy delivery technologies, and expanding indications, such as bridge to transplant in HCC or oligometastatic liver disease, may increase future utilization of liver SABR.
References
- Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312-1337.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: Cancer J Clin. 2018;68(1):7-30.
- Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3):107327481772924.
- Jarnagin W, Chapman WC, Curley S, et al. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010;12(5):302-310.
- Krenzien F, Schmelzle M, Struecker B, et al. Liver transplantation and liver resection for cirrhotic patients with hepatocellular carcinoma: comparison of long-term survivals. J Gastrointes Surg. 2018. Jan 23. [Epub ahead of print]
- Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol, 2017;67(1):92-99.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology.2007;132(7):2557-2576.
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34(5):452-459.
- Sapir E, Tao Y, Schipper MJ, et al. Stereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(1):122-130.
- Xi M, Zhang L, Zhao L, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS ONE, 2013;8(5):e63864.
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84-96.
- Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: A retrospective dose response analysis. J Clin Oncol. 2016;34(3):219-226.
- Tse, RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26(4):657-664.
- Sheth KR, Clary BM. Management of hepatic metastases from colorectal cancer. Clin Colon Rectal Surg. 2005;18(03):215-223.
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8(6):378-382.
- Van Cutsem E, Cervantes A, Adam Ret al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386-1422.
- Comito T, Cozzi L, Clerici E. Stereotactic ablative radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: a safe and effective approach. BMC Cancer, 2014;14(1):619.
- Scorsetti M, Comito T, Tozzi A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2014;141(3):543-553.
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II Trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572-1578.
- Fode MM, Hoyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol. 2015;114(2):155-160.
- Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9).
- Stintzing S, Grothe A, Hendrich S, et al. Percutaneous radiofrequency ablation (RFA) or robotic radiosurgery (RRS) for salvage treatment of colorectal liver metastases. Acta Oncologica. 2013;52(5):971-977.
- Ahmed KA, Caudell JJ, El-Haddad G, et al. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95(5):1399-1404.
- RTOG Foundation. RTOG 1112 protocol information. www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1112. Accessed January 30, 2018,
- Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol. 2006;45(7):856-864.
- Lasley FD, Mannina EM, Johnson CS, et al. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5(5):e443-449.
- Ten Haken RK, Balter JM, Marsh LH, et al. Potential benefits of eliminating planning target volume expansions for patient breathing in the treatment of liver tumors. Int J Radiat Oncol Biol Phys. 1997;38(3):613-617.
- Wagman R, Yorke E, Ford E, et al. Respiratory gating for liver tumors: use in dose escalation. Int J Radiat Oncol Biol Phys. 2003;55(3):659-668.
- Eccles CL, Patel R, Simeonov AK, et al. Comparison of liver tumor motion with and without abdominal compression using cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2011;79(2):602-608.
- Dawson LA, Brock KK, Kazanjian S, et al. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51(5):1410-1421.
- Law AL, Ng WT, Lee MC, et al. Treatment of primary liver cancer using highly-conformal radiotherapy with kV-image guidance and respiratory control. Radiother Oncol. 2012;102(1):56-61.
- Winter JD, Wong R, Swaminath A, Chow T. Accuracy of robotic radiosurgical liver treatment throughout the respiratory cycle. Int J Radiat Oncol Biol Phys. 2015;93(4):916-924.
- Kothary N, Heit JJ, Louie JD, et al. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Interv Radiol. 2009;20(2):235-239.
- Wunderink W, Méndez Romero A, Seppenwoolde Y, et al. Potentials and limitations of guiding liver stereotactic body radiation therapy set-up on liver-implanted fiducial markers. Int J Radiat Oncol Biol Phys. 2010;77(5):1573-1583.
- Yue J, Sun X, Cai J, et al. Lipiodol: a potential direct surrogate for cone-beam computed tomography image guidance in radiotherapy of liver tumor. Int J Radiat Oncol Biol Phys. 2012;82(2):834-841.
- Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24(3):196-199.
- Kishan AU, Cao M, Wang PC, et al. Feasibility of magnetic resonance imaging-guided liver stereotactic body radiation therapy: A comparison between modulated tri-cobalt-60 teletherapy and linear accelerator-based intensity modulated radiation therapy. Pract Radiat Oncol. 2015;5(5):330-337.
- Wojcieszynski AP, Rosenberg SA, Brower JV, et al. Gadoxetate for direct tumor therapy and tracking with real-time MRI-guided stereotactic body radiation therapy of the liver. Radiother Oncol. 2016;118(2):416-418.
- Hong TS, Wo JY, Borger DR, et al. Phase II study of proton-based stereotactic body radiation therapy for liver metastases: importance of tumor genotype. J Natl Cancer Inst. 2017;109(9).
- Petersen JB, Lassen Y, Hansen AT, et al. Normal liver tissue sparing by intensity-modulated proton stereotactic body radiotherapy for solitary liver tumours. Acta Oncol. 2011;50(6):823-828.
- Hong TS, DeLaney TF, Mamon HJ et al. A prospective feasibility study of respiratory-gated proton beam therapy for liver tumors. Pract Radiat Oncol. 2014;4(5):316-22.
- Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115(23):5499-5506.
- El Naqa I, Cuneo K, Owen D, et al. Radiation sensitivity of the liver: models and clinical data. In: Meyer J, Schefter T, eds. Radiation therapy for liver tumors. 1st ed. Cham, Switzerland: Springer International Publishing. 2017:39-47.
- Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S94-100.
- Klein J, Dawson LA, Jiang H, et al. Prospective longitudinal assessment of quality of life for liver cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(1):16-25.
- Huertas A, Baumann AS, Saunier-Kubs F, et al. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol. 2015;115(2):211-216.
- Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncologica. 2013;53(3):399-404.
- Yoon SM, Lim YS, Park MJ, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS ONE. 2013;8(11):e79854.
- Bibault JE, Dewas S, Vautravers-Dewas C, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS ONE. 2013;8(10):e77472.
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase i and ii trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631-1639.
- Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e447-e453.
- O’Connor JK, Trotter J, Davis GL, et al. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012;18(8):949-954.
- Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Translat Oncol. 2010;12(3):218-225.
- Meyer JJ, Foster RD, Lev-Cohain N, et al. A phase 1 dose-escalation trial of single-fraction stereotactic radiation therapy for liver metastases. Ann Surg Oncol. 2015;23(1):218-224.
- Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78(2):486-493.
- Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27(10):1585-1591.
Citation
BO S, L P, EA M. Stereotactic ablative radiation therapy in the treatment of liver tumors. Appl Radiat Oncol. 2018;(1):17-23.
March 23, 2018