Soft-tissue sarcoma of the breast following breast-conserving therapy
Images
CASE SUMMARY
A 73-year-old woman presented with a several-month history of left chest pain. She underwent a routine workup, which was initially negative. She had a history of left-sided breast cancer 24 years ago treated with lumpectomy followed by whole-breast irradiation. Imaging demonstrated a finding in the left fourth rib.
IMAGING FINDINGS
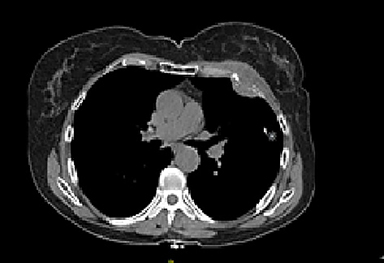
The patient underwent a computed tomography (CT) scan of the chest, which demonstrated a lytic bone lesion with a soft-tissue mass in the left fourth anterior rib (Figure 1). Mammography demonstrated a consistent finding in the posterior left breast. Positron emission tomography-CT (PET-CT) demonstrated intense hypermetabolic uptake in the lesion with no other findings noted.
DIAGNOSIS
Based on imaging and history, the differential diagnosis included metastatic breast cancer, plasmacytoma/myeloma, sarcoma, and second malignancy associated with radiation therapy. Biopsy demonstrated atypical spindle-cell proliferation consistent with spindle-cell sarcoma. The patient underwent resection with chest wall reconstruction, with final pathology demonstrating a high-grade sarcoma measuring 4.7 cm with a close deep margin (0.1 cm).
DISCUSSION
While angiosarcoma and lymphangiosarcoma are the most commonly associated second malignancies following breast radiation therapy, there is the potential for other secondary sarcomas, including bone and soft-tissue sarcomas.1,2 The standard of care for such cases remains surgery with consideration for adjuvant radiation therapy and systemic therapy for both soft-tissue and bone sarcomas based on data demonstrating improved local control and possible improvement in survival with adjuvant RT for primary high-grade soft-tissue sarcomas.3,4 However, the greatest challenge with this case was delivering re-irradiation to the left chest in the setting of previous radiation therapy and managing heart and lung dose, particularly with a close deep margin.
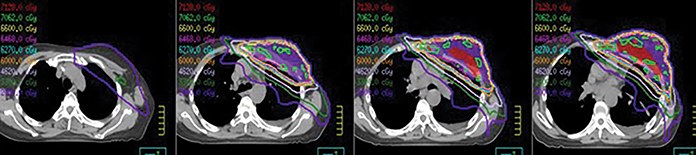
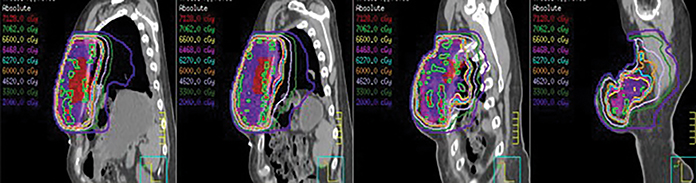
It should be noted that limited data are available with respect to repair to the heart and lungs following breast radiation therapy and subsequent dose constraints in the setting of re-irradiation. As such, we decided to optimize fractionation, treatment volumes and delivery techniques. With respect to dose and fractionation, we chose an approach to combine acceleration and hyperfractionation with a fractional dose of 1.5 Gy delivered twice daily to a total dose of 66 Gy. The goal was to reduce the risk of chronic toxicities while maintaining tumor control, understanding the potential for increased acute and subacute toxicities.5 Similarly, to reduce dose to the heart and lungs, the clinical target volume (CTV) was reduced,6 the initial imaging was fused to the treatment planning scan to allow for creation of a pre-surgical gross tumor volume (GTV). Subsequently, a CTV was created expanding 2 cm beyond the GTV with a 1 cm radial expansion while limiting the CTV expansion into the lungs and heart. A planning target volume expansion of 0.5 cm was utilized. Despite being adjacent to the heart, the intensity-modulated radiation (IMRT) plan allowed for a mean heart dose of 18.1 Gy and a mean left lung dose of 23 Gy, with much of the lung dose confined to an area already irradiated by the initial breast tangents. Image guidance was performed daily with cone-beam CT, with alignment to the reconstructed chest wall (Figures 2 and 3). During the course of radiation therapy, the patient experienced fatigue with Grade 1 radiation dermatitis and no other acute toxicities. Subsequent follow-up at 3 months demonstrated no subacute or chronic toxicities to date.
CONCLUSION
Re-irradiation can be considered for secondary sarcomas following breast cancer radiation therapy. Clinicians should take advantage of fractionation, IMRT, reduced target volumes, and image guidance to maximize local control while minimizing the risk of late toxicities.
REFERENCES
- Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer. 2001;92:172-180.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009;115:4055-4063.
- Yang JC, Change AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197-203.
- Koshy M, Riche SER, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int J Radiat Oncol Biol Phys. 2010;77:203-209.
- Hall EJ, Giaccia AJ. Time, dose, and fractionation in radiotherapy. In: Hall EJ, Giaccia AJ, eds. Radiobiology for the radiologist. 6th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2006:378-397.
- Wang D, Zhang Q, Eisenberg BL, et al. Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: results of radiation therapy oncology group RTOG -0630 trial. J Clin Oncol. 2015;33:2231-2238.
Citation
TD S, N K, C S. Soft-tissue sarcoma of the breast following breast-conserving therapy. Appl Radiat Oncol. 2016;(3):36-37.
September 12, 2016