Radiation necrosis: Now you see it, now you don't
Images
CASE SUMMARY
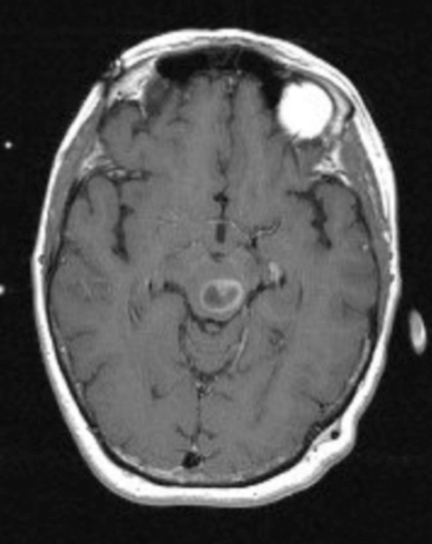
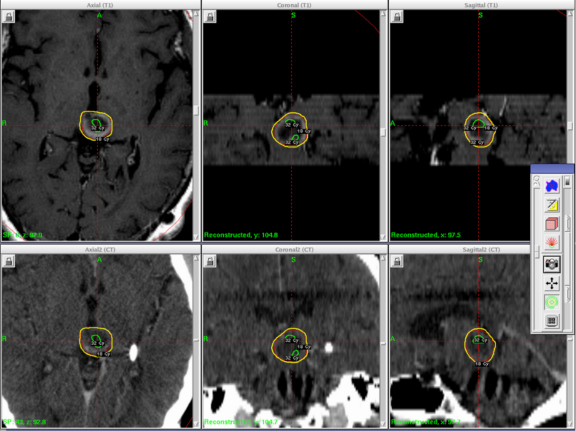
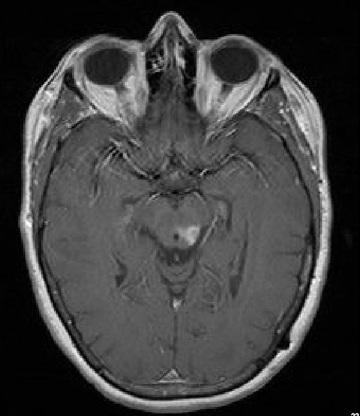
A 61-year-old woman with a large-cell neuroendocrine carcinoma of the lung, at her 10-month post-lobectomy and chemotherapy status had developed a 1.3 × 1.8 × 1.4-cm left thalamic/tectal lesion (Figure 1). Consideration was noted for metastasis, and it was treated with Gamma Knife stereotactic radiosurgery (SRS, Figure 2).
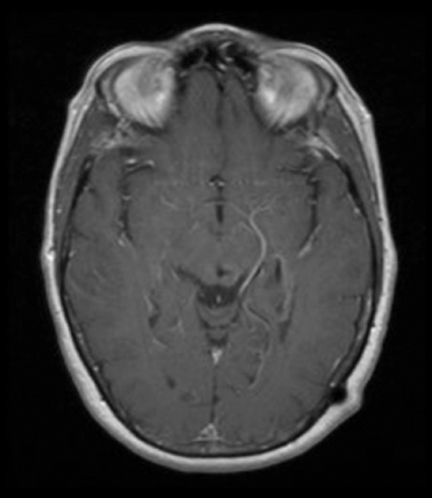
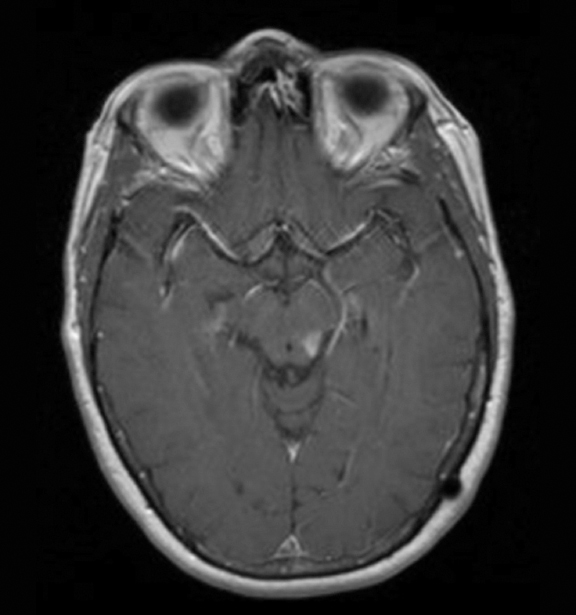
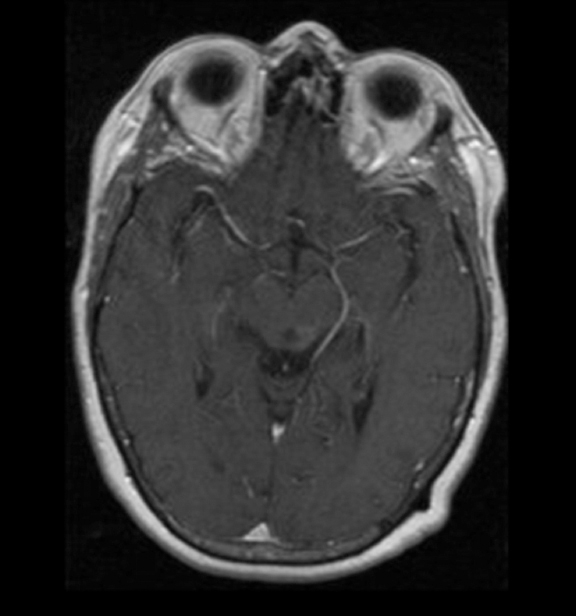
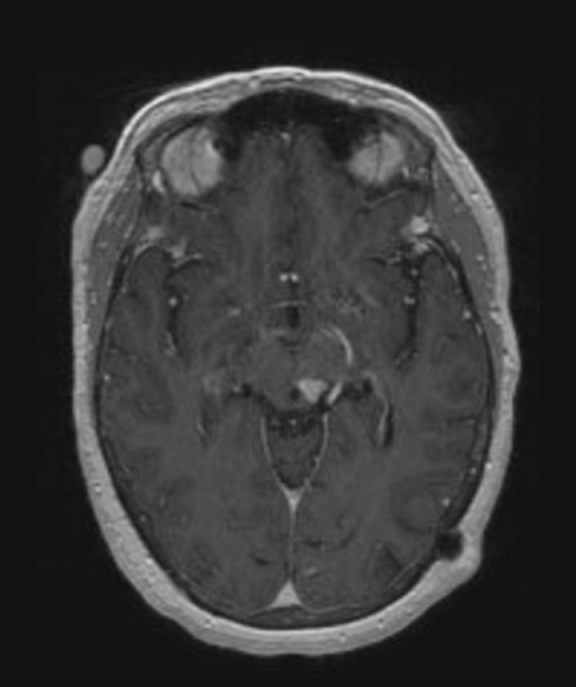
The lesion initially regressed (Figure 3). However, at 14 months post-SRS, the patient developed fatigue, right-sided hemianesthesia, thermoanesthesia and diplopia, and enhancement at the SRS site (Figure 4), which persisted despite dexamethasone treatment (Figures 5 and 6). After 4 doses of bevacizumab, the patient’s symptoms stabilized, but she experienced a generalized tonic-clonic seizure, at which time magnetic resonance imaging (MRI) demonstrated near-resolution of enhancement (Figure 7). The lesion returned to its size at onset of radiation necrosis (RN) symptoms, and remained stable through 37 months post-SRS (Figure 8).
The patient was without evidence of active disease 45 months after SRS, with stable hemianesthesia, hemithermoanesthesia, exotropia, hypertropia, and diplopia.
IMAGING FINDINGS
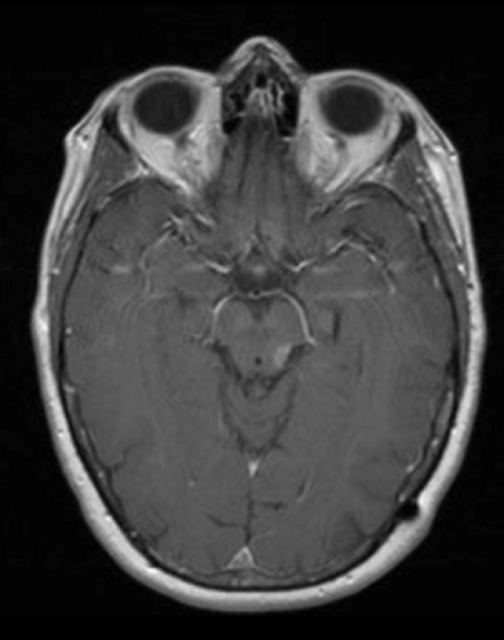
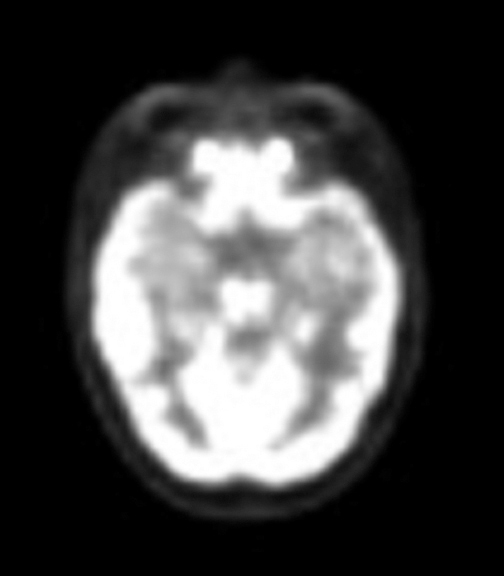
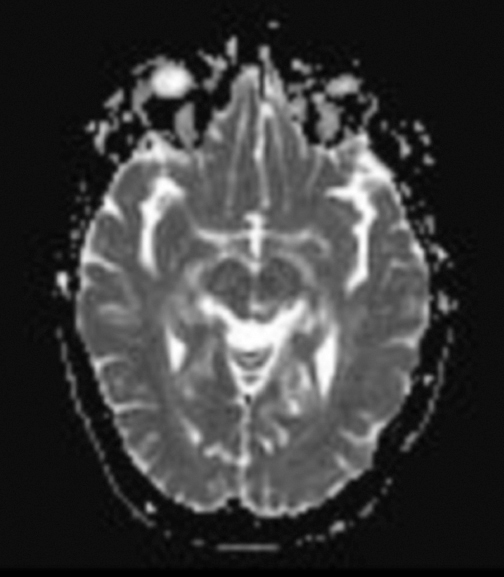
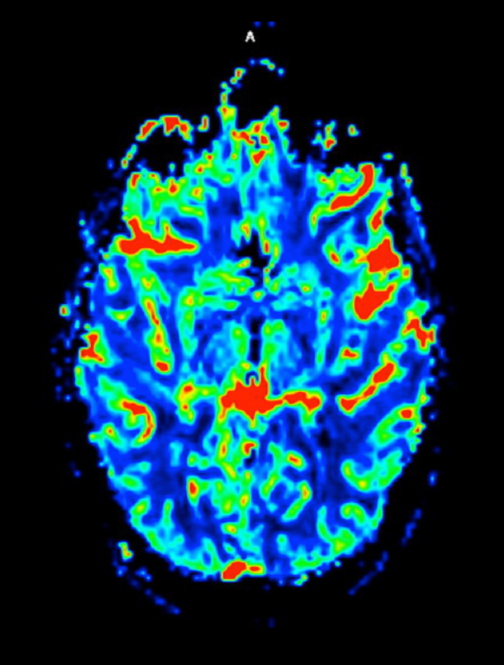
At diagnosis of presumed brain metastasis, axial T1 contrast-enhanced MRI demonstrated a 1.3 × 1.8 × 1.4-cm cystic, peripherally enhancing mass in the left thalamus/tectum causing obstructive hydrocephalus (Figure 1). At 11 months post-SRS, axial T1 contrast-enhanced MRI showed no discrete mass, mass-effect, midline shift or abnormal lesion (Figure 3). Three months later, axial T1 contrast-enhanced MRI demonstrated enhancement at the site of treatment (Figure 4). At 18 months post-SRS, axial T1 contrast-enhanced MRI showed radiographic stability (Figure 5). Advanced imaging techniques for differentiation of RN from tumor recurrence included relative cerebral blood volume (rCBV) MRI and diffusion weighted imaging (DWI) with associated apparent diffusion coefficient (ADC), demonstrated no restricted diffusion or increased perfusion (Figure 5). Fluorodeoxyglucose positron emission tomography FDG-PET) revealed focal photopenia with decreased FDG uptake at the site of SRS, consistent with radiation changes (Figure 5). T1 contrast-enhanced MRI at 18 months post-SRS demonstrated a stable, persistently enhancing lesion at the site of SRS (Figure 6). After 4 cycles (2 months) of bevacizumab (20 months post-SRS), T1 axial contrast-enhanced MRI demonstrated minimal enhancement, indicating substantial resolution of the lesion (Figure 7). At 37 months post-SRS, the left thalamic lesion was comparable in size to the MRI at 14 months post-SRS, at which point the patient had become symptomatic (Figure 8).
DIAGNOSIS
Differential diagnosis included RN, tumor progression, or mixed RN/tumor progression
DISCUSSION
As reviewed in a previous case study, diagnosing RN is complicated.1 The only aspect of RN more controversial than accurately diagnosing RN is its management.
Since first discussed in the late 1980s and early 1990s, more than 35 years after pioneering SRS and 60 years after the first case of intracranial RN. In the 30 years since the initial reports, SRS use has increased, but treatment of RN remains nearly as perplexing now as it was then.
Current literature supports the findings of case reports from the 1930s and 1940s, which described clinical courses varying from indolent symptom development to rapidly progressive, fatal courses. Asymptomatic, or radiographic, RN may also occur.
Predictive models and parameters have been developed to reduce the risk of post-SRS RN, but once diagnosed, its treatment remains controversial, as a paucity of data exists regarding RN management.
Published rates of clinical, or symptomatic, RN vary, but may range from 10% to14%. Asymptomatic, or radiographic RN, may occur in 14% to 50% of patients.1,2 Patients with RN are often treated with corticosteroids, which disrupt the blood-brain barrier, impact VEGF, demonstrate anti-inflammatory effects, and modulate vasodilation, all of which have been linked to the pathophysiology of RN. Patients with RN often demonstrate vasogenic edema, which may result in increased intracranial pressure, focal neurologic symptoms, and seizures. Corticosteroids may bridge these patients through a self-limiting process or they may have therapeutic benefit.3-5
Surgery provides histopathologic confirmation and, in many cases, offers sufficient therapeutic intervention. However, not all lesions are amenable to surgical intervention. Noninvasive treatments explored in the treatment of RN include anticoagulation, non-steroidal anti-inflammatory agents (NSAIDs), pentoxifylline with or without vitamin E, bevacizumab, and hyperbaric oxygen therapy (HBOT).
Pentoxifylline with vitamin E was reported in a post-SRS RN case series in which 10 of 11 patients demonstrated volumetric reduction in RN-related edema.6 The patient without response was subsequently diagnosed with tumor recurrence. No clinical correlation was provided.
One study of 101 brain metastases in 78 patients randomized to post-SRS HBOT demonstrated radiation injury in 11% of HBOT patients and 20% of observation patients.7 HBOT decreased rates of white matter injury, but not RN incidence.
Bevacizumab (Avastin®) was studied prospectively in a randomized, controlled trial in patients previously treated with fractionated radiotherapy.8 All patients randomized to bevacizumab, and all cross-over patients, demonstrated clinicoradiographic response to bevacizumab. At 10 months of follow up, 2 of 12 patients analyzed demonstrated radiographic changes consistent with RN recurrence.
Our patient experienced progressive symptoms and radiographic findings despite corticosteroids. Bevacizumab was administered, with dose escalation after 2 cycles for minimal clinicoradiographic response. After 4 cycles, the patient experienced a generalized tonic-clonic seizure and bevacizumab was discontinued. MRI at that time demonstrated resolution of enhancement. Follow-up imaging demonstrated recurrence of enhancement at the site of SRS. The patient currently has persistent, stable radiographic enhancement with right hemianesthesia, right hemithermoanesthesia, and diplopia.
It is unclear whether our patient would have had clinicoradiographic progression without pharmacologic intervention. The lesion may have stabilized after onset of symptoms at 18 months post-SRS. Anecdotal experiences at our institution support case reports denoting varied clinicoradiographic courses of RN.9,10 Escalation of therapeutic interventions through corticosteroids (without standard “trial” duration), additional pharmacotherapies, HBOT, and surgical intervention is not well established.
The pathogenesis of RN of the brain is not well-understood. No prospective, randomized controlled trials exist regarding post-SRS RN. Interventions remain based on agents effective at mitigating radiation effects in other areas of the body as understanding of post-SRS and its treatment continues to develop.
REFERENCES
- Stockham AL. Chao St, Suh JH. Wanted: Dead or alive? Distinguishing radiation necrosis from tumor progression after stereotactic radiosurgery. Applied Radiology. 2012;1:26-29.
- American Cancer Society. www.cancer.org/Research/CancerFactsFigures/index. Accessed August 10, 2012.
- Martins AN, Severance RE, Henry JM, Doyle TF. Experimental delayed radiation necrosis of the brain Part 1: Effect of early dexamethasone treatment. J Neurosurg. 1979;51:587-596.
- Shaw PJ, Bates D. Conservative treatment of delayed cerebral radiation necrosis. J Neurol Neurosurg Psychiatry. 1984;47:1338-1341.
- Tada E, Matsumoto K, Kinoshita K, Furuta T, Ohmoto T. The protective effect of dexamethasone against radiation damage induced by interstitial irradiation in normal monkey brain. Neurosurgery. 1997;41:209–217.
- Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008;86:359-366.
- Ohguri T, Imada H, Kohshi K, Kakeda S, Ohnari N, Morioka T, Nakano K, Konda N, Korogi Y. Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. 2007;67:248-255.
- Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487-1495.
- Sanborn MR, Danish SF, Rosenfeld MR, O’Rourke D, Lee JY. Treatment of steroid refractory, Gamma Knife related radiation necrosis with bevacizumab: case report and review of the literature. Clin Neurol Neurosurg. 2011;113:798-802.
- Jeyaretna DS, Curry WT Jr, Batchelor TT, Stemmer-Rachamimov A, Plotkin SR. Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol. 2011; 29:159-162.