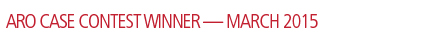Radiation-induced pathologic complete response of gross nodal disease in recurrent head and neck melanoma
Images

CASE SUMMARY
A 52-year-old male presented with a 4-by-2-mm brown macule on the central midline of his forehead; it had reticulated edges, which had been present for 1 year. A shave biopsy diagnosed lentigo maligna melanoma with tumor thickness of 1.5 mm, Clark level 3. The patient underwent staging sentinel lymph node mapping with TC-99M scintigraphy. He proceeded with wide local excision and sentinel lymph node biopsy, with pathology negative for residual disease. No lymph nodes were identified; thus, initial AJCC Stage pT2N0M0 was diagnosed.
At 5 months follow-up, a 2 cm firm left submandibular lymph node was noted on exam. Fine-needle aspiration favored recurrent melanoma. A staging positron emission tomography/computed tomography (PET/CT) scan showed 2 enlarged lymph nodes adjacent to the left submandibular gland measuring 3.4-by-2.5 cm (SUV of 3.5) and 2.1-by-1.6 cm (SUV of 4.4). The patient underwent left neck dissection of levels IB, II and III with 9.0-by-4.5-by-1.7 cm of tissue removed and 14 total lymph nodes removed with only 1 positive for disease. ENT notes indicated that the left submandibular gland was preserved. There was no evidence of extracapsular extension. He received postoperative radiation given recurrent nodal disease. An enlarged level Ib lymph node was seen on post-op imaging obtained for radiation planning. Radiation entailed 3000 cGy in 5 fractions delivered twice weekly over 14 days. A planned left submandibular nodal dissection was performed 7 weeks after the completion of radiation, with pathology reporting evidence of regressed melanoma and no viable tumor. He had no postoperative complications or difficulty with wound healing. A restaging PET/CT and exam showed no recurrent disease 3 months after therapy.
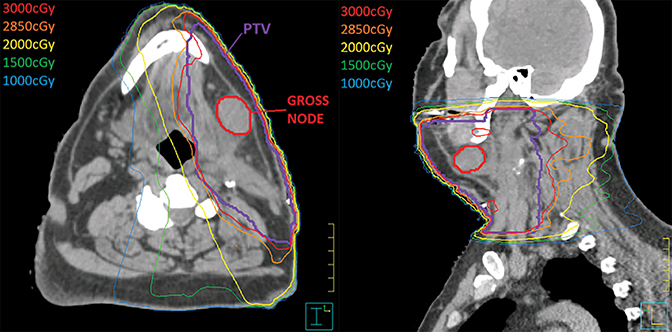
IMAGING FINDINGS AND DIFFERENTIAL DIAGNOSIS
Initial preoperative PET/CT (Figure 1) demonstrated moderate hyper-metabolism of 2 adjacent masses within the left neck near the left submandibular gland. These are suspicious for potential level 1 lymph node metastases associated with the patient’s melanoma. The differential diagnosis would include metastases associated with a second primary head and neck neoplasm.
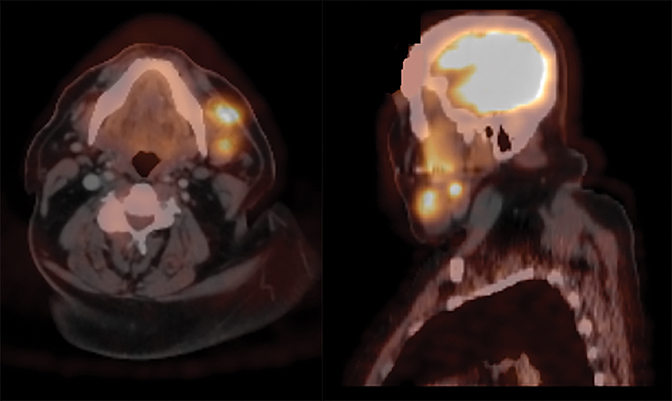
Postoperative CT used for RT planning (Figure 2) demonstrated persistence of a single mass near the left submandibular gland. Seven weeks after radiation, path slides (Figure 3) showed irradiated lymph node with necrosis, fibrosis, and residual heavy pigment consistent with a regressed tumor (pCR).
DIAGNOSIS
Recurrent head and neck melanoma
DISCUSSION
The optimal management of regional nodal disease in melanoma is controversial.
For intermediate thickness (1.0 mm to 4.0 mm) melanomas, sentinel lymph node biopsy (SLNB) is advocated as the standard management with regional nodal dissection reserved for stage III disease and considered if SLNB is positive.1,2 Despite the utility of SLNB providing staging information that is helpful for adjuvant treatment decisions, overall survival benefit was not demonstrated in a large randomized study.3 In the setting of a negative sentinel lymph node, elective dissection is not recommended given no overall survival benefit in 4 early randomized surgical trials.4-7
Adjuvant radiation therapy should be considered for patients with possible residual microscopic disease to improve local control.2 Increased risk of microscopic residual disease is often estimated with the presence of the following pathologic features: primary lesions > 4 mm, satellitosis, desmoplastic subtype, presence of 1 or more parotid lymph nodes of any size, lymph nodes ≥ 3 cm, extranodal extension, multiple nodes, recurrent disease, and close or positive margins.8-14
A phase III randomized multi-institutional study was conducted by the Trans-Tasman Radiation Oncology Group (TROG), evaluating the benefit of adjuvant radiation vs. observation after therapeutic lymphadenectomy for melanoma.14 Eligible patients were considered high risk for regional relapse due to large lymph nodes, multiple involved nodes, and/ or extracapsular extension. Eligibility criteria differed depending on nodal site (ie., parotid, cervical, axillary, etc.) for lymph node size and number. Radiation consisted of 48 Gy/20 fractions within 12 weeks following surgery. At a median of 3 years, local control within the nodal basin was improved with radiation, 82% vs. 69% (p = 0.041), HR of 0.56. Overall survival and rate of distant metastasis were similar. Toxicity was low for patients treated with neck radiation (3% grade 3 or 4 dermatitis). Sentinel lymph node biopsy was not routinely performed within the study, and patients with recurrent disease were not included.14
The ideal radiation fraction size for melanoma remains unknown. Early radiobiological data from Dewey15 showed that melanoma cells in vitro had broad shoulders on survival curves, which suggested that hypofractionated radiation would induce higher response rates compared to standard fractionation. MD Anderson Cancer Center (MDACC) introduced an adjuvant hypofractionated radiation regimen, 6 Gy x 5 fractions for subclinical disease, and 6 Gy x 6 for gross disease, with promising results from their phase II trial showing 88% locoregional control at a median follow-up of 3 years.16,17 This regimen was delivered twice weekly over 2.5 weeks, while limiting spinal cord, brain and small bowel to 24 Gy, when treating subclinical disease. However, a randomized clinical study (RTOG 83-05) disputed the laboratory data given no significant difference in response comparing 32 Gy/4 fractions vs. 50 Gy/20 fractions.18 The University of Florida performed a retrospective comparison of 30 Gy/5 fractions vs. 60 Gy/30 fractions, showing no significant difference in local control, 87% vs. 78% respectively, when treating subclinical disease. Hypofractionation was advocated given the benefit of a shorter treatment time, unless the cosmetic and/or functional outcome could be compromised.19,20
Hypofractionated radiotherapy regimens for melanoma of the head and neck have been primarily utilized in the adjuvant setting to reduce local regional relapse rates. This is the first case report we are aware of demonstrating histologic confirmation of a pathological complete response (pCR) for gross cervical nodal disease following hypofractionated radiation. A small (n=12) retrospective study evaluated neoadjuvant radiation in patients with locally advanced axillary, inguinal or popliteal metastatic melanoma.21 No patients with head and neck disease were included in the study. Forty-eight Gy in 20 fractions was the most common radiation regimen (8/12 patients) with the remaining 4 patients receiving different schedules (30 Gy/6, 32 Gy/8, 36 Gy/9, 50 Gy/20 fractions). Node dissection was performed in 10/12 patients with 9 samples available for histologic response. There were 2 patients (22%) with pCR, 5 with pPR, and 2 with no evidence of treatment response. One year in-field control was 92%. Overall, this treatment strategy was well-tolerated, with 4 patients developing minor wound complications.21
MDACC reported excellent regional control for patients who underwent radiation in lieu of completing neck dissection for melanoma of the head and neck. In a retrospective review (n=36), patients underwent excision of the primary cutaneous melanoma and any clinically apparent lymphadenopathy. No formal neck dissections were performed. Hypofractionated radiation (30 Gy in 5 fractions for elective disease) was delivered to the nodal basin with locoregional control rates of 93% at 5 years.22
Long-term toxicities associated with hypofractionated radiation appear tolerable. MDACC reported 5-year rates of grade 1 toxicity of 12% (atrophy, loss of subcutaenous fat), and grade 2 toxicity of 10% (functional deficits and/or long-term pain).23 University of Florida described 2 patients with long-term toxicity after 30 Gy/5 fractions with 1 case of osteoradionecrosis of the external auditory canal and 1 case of plexopathy.19 Long-term lymphedema risk is low after head and neck radiation, but higher rates have been reported for inguinal node irradiation.24 Some advocate conventional fractionation for disease near optic structures, spinal cord, or brainstem to prevent long-term sequelae. If using hypofractionated RT, neurologic structures are generally limited to 24 Gy.17
Radiation has not demonstrated any survival benefit in treating melanoma given the high rate of distant metastatic recurrences. However, increasingly effective systemic agents such as ipilimumab25 and vemurafenib (in patients with candidate genetic BRAF mutations)26 have demonstrated overall survival benefits. Better distant disease control and further understanding of the interaction of radiation with the immune system27 may lead to an expanded role of radiation therapy in melanoma treatment.
CONCLUSION
Current guidelines for head and neck melanoma with localized nodal disease include neck dissection with or without adjuvant radiation depending on pathologic risk factors. Adjuvant radiation offers improvement in local control; however, no effect on overall survival has been demonstrated. The current goal of radiation therapy is to improve locoregional control through prevention of recurrences and the associated morbidity of local progression including pain, ulceration, bleeding, disfigurement, and the need for additional surgery.
In this case, hypofractionated radiation induced a pathological complete response in recurrent gross nodal disease. This case reinforces the efficacy of 30 Gy hypofractionated radiation therapy for localized nodal melanoma. This regimen could be considered for future study in the neoadjuvant setting, or as a potential definitive therapy for inoperable patients.
REFERENCES
- Wong SL, Balch CM, Hurley P, et al. Sentinel lymph node biopsy for melanoma: American society of clinical oncology and society of surgical oncology joint clinical practice guideline. J Clin Oncol. 2012;30(23):2912-2918. doi: JCO.2011.40.3519.
- Coit DG, Thompson JA, Andtbacka R, et al. Melanoma, version 4.2014. J Natl Compr Canc Netw. 2014;12(5):621-629. doi: 12/5/621 [pii].
- Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599-609.
- Veronesi U, Adamus J, Bandiera D, et al. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982;49(11):2420-2430.
- Sim, FH, Taylor, WF, Pritchard, DJ, et al. Lymphadenectomy in the management of stage I malignant melanoma: a prospective randomized study. Mayo Clin Proc.1986;61(9):697-705.
- Cascinelli N, Morabito A, Santinami M, et al. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. The Lancet. 1998;351(9105):793-796.
- Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Ann of Surg Oncol. 2000;7(2):87-97.
- Leon P, Daly JM, Synnestvedt M, et al. The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg. 1991;126(12):1461-1468.
- Calabro A, Singletary SE, Balch CM. Patterns of relapse in 1001 consecutive patients with melanoma nodal metastases. Arch Surg. 1989;124(9):1051-1055.
- Shen P, Wanek LA, Morton DL. Is adjuvant radiotherapy necessary after positive lymph node dissection in head and neck melanomas? Ann Surg Oncol. 2000;7(8):554-559.
- Lee RJ, Gibbs JF, Proulx GM, et al. Nodal basin recurrence following lymph node dissection for melanoma: Implications for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46(2):467-474.
- Vuylsteke RJ, van Leeuwen PA, Statius Muller MG, et al. Clinical outcome of stage I/II melanoma patients after selective sentinel lymph node dissection: long-term follow-up results. J Clin Oncol. 2003;21(6):1057-1065.
- Ballo MT, Ang KK. Radiotherapy for cutaneous malignant melanoma: rationale and indications. Oncol. 2004;18(1):99-107.
- Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. The Lancet Oncol. 2012;13(6):589-597.
- Dewey DL. The radiosensitivity of melanoma cells in culture. Br J Radiol. 1971;44(526):816-817.
- Ang K, Byers R, Peters L, et al. Regional radiotherapy as an alternative or adjuvant to nodal dissection for high risk cutaneous malignant melanoma of the head and neck. Arch Otolaryngol Head Neck. 1990;116:169-172.
- Ang KK, Peters LJ, Weber RS, et al. Postoperative radiotherapy for cutaneous melanoma of the head and neck region. Int J Radiat Oncol Biol Phys. 1994;30(4):795-798.
- Sause W, Cooper J, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. 1991;20(3):429-432.
- Chang DT, Amdur RJ, Morris CG, et al. Adjuvant radiotherapy for cutaneous melanoma: Comparing hypofractionation to conventional fractionation. Int J Radiat Oncol Biol Phys. 2006;66(4):1051-1055.
- Mendenhall WM, Shaw C, Amdur RJ, et al. Surgery and adjuvant radiotherapy for cutaneous melanoma considered high-risk for local–regional recurrence. Am J Otolaryngol. 2013;34(4):320-322.
- Foote M, Burmeister B, Dwyer P, et al. An innovative approach for locally advanced stage III cutaneous melanoma: radiotherapy, followed by nodal dissection. Melanoma Res. 2012;22(3):257-262. doi: 10.1097/CMR .0b013e3283531335 [doi].
- Ballo MT, Garden AS, Myers JN, et al. Melanoma metastatic to cervical lymph nodes: Can radiotherapy replace formal dissection after local excision of nodal disease? Head Neck. 2005;27(8):718-721.
- Ballo MT, Bonnen MD, Garden AS, et al. Adjuvant irradiation for cervical lymph node metastases from melanoma. Cancer. 2003;97(7):1789-1796.
- Guadagnolo BA, Zagars GK. Adjuvant radiation therapy for high-risk nodal metastases from cutaneous melanoma. Lancet Oncol. 2009;10(4):409-416.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-2516.
- Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;88(5):986-997.