Proton therapy for lung cancer: Current uses and future applications for early stage and locally advanced non-small cell lung ca
Images
Lung cancer remains the leading cause of cancer death in the United States. In 2016, an estimated 224,390 new cases of lung cancer and 158,080 deaths related to lung cancer will occur.1 Of those diagnosed, approximately 85% will be the non-small cell histologic type. Non-small cell lung cancer (NSCLC) has a very poor prognosis, with 5-year survival rates of approximately 18% across stages. Over 55% of patients are diagnosed with stage IV disease, and these patients have a particularly poor survival of only approximately 4% at 5 years.2,3
For patients with localized or regional NSCLC, radiation therapy is often a part of or the primary mode of treatment, with approximately 60% of patients receiving radiation.4 In early stage disease, radiation therapy is used as definitive monotherapy most commonly in patients who are medically inoperable or refuse surgery.5 In locally advanced disease, radiation therapy is given as bimodality definitive therapy concurrently with or sequential to chemotherapy, or it is delivered as part of trimodality therapy among patients with resectable disease.6
Although treatment has become more precise, toxicity associated with thoracic radiation therapy, particularly when combined with chemotherapy, remains significant. For instance, in RTOG 0617,7 which compared 60 Gy to 74 Gy with concurrent chemotherapy in the treatment of stage III NSCLC, 76% to 79% of patients developed grade ≥ 3 toxicity. Overall survival was inferior in the dose escalation arm, which was attributed, in part, to the high incidence of high-grade esophagitis and the higher heart doses delivered in patients receiving 74 Gy.
Treatment of NSCLC is particularly challenging since the dose needed to kill the tumor is often higher than the tolerance of the surrounding critical structures. Toxicities to healthy lung parenchyma and surrounding critical organs such as the heart, esophagus, bronchial tree, spinal cord, and brachial plexus can all be experienced with radiation therapy.8 Compounding the issue, most lung cancer patients have a smoking history and often have pulmonary and/or cardiac disease, making them more susceptible to radiation therapy toxicities. The ideal radiation treatment plan is one that delivers a tumoricidal dose while limiting dose to normal tissue.
Proton Therapy for Lung Cancer
Unlike photon-based radiation therapy, which delivers dose throughout the course of the beam path, the physical properties of proton therapy allow for energy to be deposited at a specific depth, also called the Bragg peak. Distal to this depth, a rapid energy falloff is achieved, which allows normal tissues beyond the tumor depth to receive little or no dose of irradiation. This property gives protons better dose distributions, thus limiting dose to nearby critical structures.
In lung cancer, proton therapy can minimize dose to lung and surrounding structures, which might allow for reduced treatment toxicities. This can also allow for the treatment of tumors close to critical structures and for dose escalation,9 which may result in better local tumor control.10 Decreased dose to nearby healthy tissue also allows for potentially safer use of multimodality therapy and the possibility of reirradiation in the setting of local or regional recurrence.11
Early Stage NSCLC
The mainstay of treatment for early stage NSCLC is surgery, with a survival rate of 60% to 80% at 5 years.12 However, many patients are not optimal surgical candidates due to age, poor cardiopulmonary function or other medical comorbidities, or they elect not to pursue definitive surgical management. In these patients, radiation therapy is the recommended treatment of choice. Hypofractionation and stereotactic body radiation therapy (SBRT), also termed stereotactic ablative radiation therapy (SABR), have comparable clinical outcomes to surgery for early stage NSCLC and generally more favorable clinical and toxicity outcomes compared with conventionally fractionated radiation schedules.13,14 However, SBRT generally utilizes multiple beams or arcs, exposing larger volumes of lung to lower doses of radiation, which can result in pulmonary toxicity.15 This makes the delivery of SBRT in patients who already have severe respiratory disease challenging. Additionally, studies have shown a higher risk of toxicities to the bronchial tree, vasculature, and surrounding critical structures when SBRT is used to treat central or ultracentral lesions.16,17 Dose to the PTV has to be dialed back in some cases in order to avoid toxicities.18
The benefit of protons over photons for early stage NSCLC has been demonstrated in several planning studies19,20 and retrospective studies in improving tumor coverage and/or reducing dose to the lungs, heart, esophagus, and spinal cord.
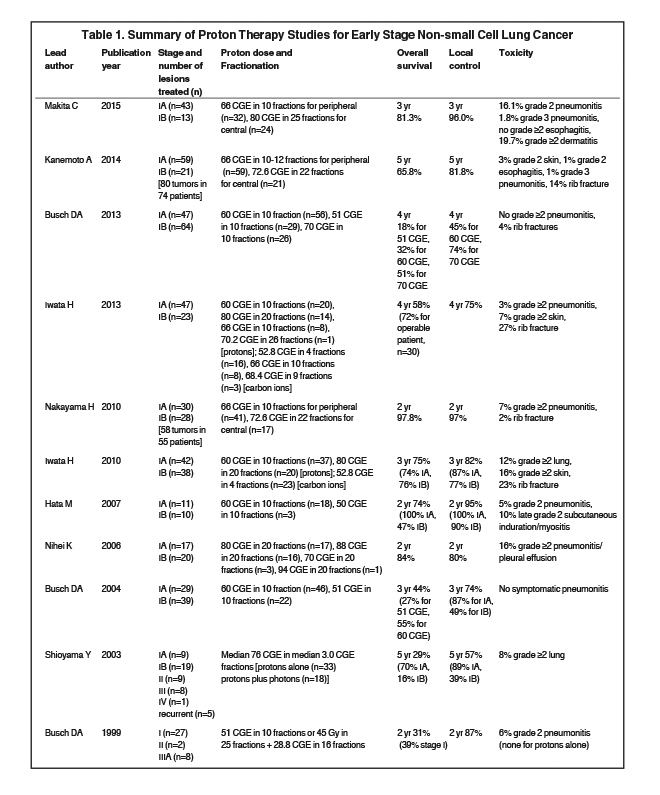
Many clinical studies have used protons to treat early stage NSCLC (Table 1)
. In 1999, an early prospective study was published by Loma Linda21 in which 27 patients with early stage disease were assigned to one of two arms. Patients with adequate cardiopulmonary function received 45 Gy of photon therapy in 25 fractions to the GTV and mediastinum with a proton boost of 28.8 CGE (cobalt gray equivalents) in 16 fractions. Patients with poor cardiopulmonary function received 51 CGE of proton therapy in 10 fractions just to the GTV. At 2 years, disease-free survival was 86% and local disease control was 87%. Toxicities were minimal, with no grade > 2 esophagitis, and only 2 patients developing clinical radiation pneumonitis, both of whom had grade 2 pneumonitis that resolved with oral steroids.
Recognizing the higher rates of recurrence in patients who receive conventionally fractionated radiation therapy and the normal tissue dose constraints that limit dose escalation with photons, Loma Linda performed a phase II trial that treated stage I patients who refused surgery or were medically inoperable with hypofractionated proton therapy.22 Twenty-two patients were treated with 51 CGE in 10 fractions over two weeks and 46 were treated with 60 CGE on the same schedule. At 3 years, local tumor control was 74% and disease-specific survival was 72%. A significant improvement in 3-year survival was noted for patients receiving 60 CGE (55% vs. 27%, p = 0.03). No cases of clinical acute radiation pneumonitis, acute or late esophageal toxicity, or cardiac toxicity were seen. In an updated report23 in which 111 patients were treated with 51, 60 or 70 CGE of hypofractionated protons, the 4-year overall survival was 18%, 32%, and 51%, respectively (p = 0.006). For T1 tumors, 4-year local control was 86% with 60 Gy, and 91% with 70 Gy. A more notable difference was seen with a higher dose for T2 disease, with a 4-year local control of 45% with 60 Gy, and 74% with 70 Gy. There were no toxicities of grade 2 or worse.
At the University of Tsukuba in Japan, an early retrospective study reported on 28 patients with stage I disease treated with hypofractionated protons. The total equivalent doses were a median of 75.0 Gy for stage IA disease and 87.8 for IB disease.24 Five-year overall survival was 70% for 9 stage IA patients and 16% for 19 stage IB patients, whereas 5-year in-field local control was 89% for IA and 39% for IB patients. In a prospective study from University of Tsukuba, 21 patients with stage I disease were treated with hypofractionated protons to 50 or 60 Gy.25 At 2 years, local progression-free rate was 100% and 90%, overall survival was 100% and 47%, and cause-specific survival was 100% and 70%, for stage IA and IB disease, respectively. There were no grade ≥ 3 toxicities, and only 1 patient developed a grade 2 pneumonitis. In a 2010 expanded analysis of 55 patients with stage I disease treated to 72.6 Gy in 22 fractions to central lesions and 66 Gy in 10 fractions to peripheral lesions, at 2 years, overall survival, progression-free survival, and local control were 97.8%, 88.7% and 97%, respectively.26 In an updated 2014 report, 74 patients with 80 lesions were treated to 72.6 Gy in 22 fractions for central tumors and 66 Gy in 10 or 12 fractions for peripheral tumors.27 At 3 years, overall survival was 76.7%, disease-specific survival was 58.6%, and progression-free survival was 58.6%. The 3-year local control was 86.3% for stage IA and 67% for stage IB, and it was 88.4% for peripheral lesions and 63.9% for central lesions. There was 1 case of grade 3 pneumonitis and 11 cases of grade 4 rib fractures.
In a retrospective report from National Cancer Center East, 17 patients with stage IA disease and 20 with IB disease were treated to 70-94 Gy in 20 fractions.28 At 2 years, local progression-free survival was 80% and overall survival was 84%. For IA and IB disease, locoregional relapse-free survival rates were 79% and 60%, respectively.
In 2010, Iwata et al29 published a report in which patients with stage I disease were treated with protons or carbon ions. Fifty-seven patients were treated with protons in 20 fractions to 80 Gy or 10 fractions to 60 Gy. At 3 years, overall survival was 90% and 61%, and local control was 83% and and 81% for 80 Gy and 60 Gy, respectively. In a 2013 report by Iwata el al treating larger tumors (T2A and T2B) with protons or carbon ions, the 4-year overall survival was 58%.30
In a recent retrospective report by Makita et al,31 32 patients with peripheral tumors were treated in 10 fractions to 66 Gy (6.6 Gy/fraction) and 24 patients with central tumors were treated in 25 fractions to 80 Gy (3.2 Gy/fraction). At 3 years, overall survival, progression-free survival and local control were 81.3%, 73.4% and 96%, respectively, with no significant differences between dosing regimens. No grade 4 or 5 toxicities were observed, and grade 3 toxicities were limited to a single patient (1.8%) with dermatitis and a single patient with pneumonitis.
Locally Advanced NSCLC
Dosimetric and clinical studies have demonstrated potential advantages of protons over photons in the treatment of locally advanced NSCLC. Chang et al32 compared dose-volume histograms for protons and photons and found that protons delivered less dose to the lungs, spinal cord, heart and esophagus compared to photons (both 3-dimensional conformal radiation therapy [3D-CRT] and intensity-modulated radiotherapy [IMRT]).
A phase II study performed at MD Anderson Cancer Center enrolled 44 patients with stage III NSCLC.33 Treatment was to 74 Gy with proton therapy at 2 Gy/fraction with concurrent carboplatin and paclitaxel. The median overall survival was 29.4 months. At 1 year, overall survival was 86% and progression-free survival was 63%. There were no grade 4-5 radiation toxicities. Grade 3 toxicities included 5 cases of dermatitis, 5 cases of esophagitis, and only 1 case of radiation pneumonitis. Of note, the median overall survival for stage III patients in RTOG 0117, in which they were treated with 74 Gy of photons plus concurrent carboplatin-paclitaxel, was relatively lower at 21.6 months,34 similar to the 20.3 months for the 74 Gy concurrent carboplatin-paclitaxel photon radiation therapy arm of RTOG 0617.7 In an expanded report of 84 patients, the median survival was 29.9 months. At 3 years, progression-free survival was 31.2% and overall survival was 37.2%.35 In their 2015 report of their prospective observational study, MD Anderson investigators treated 134 patients with stage II-III NSCLC with passive scattering proton therapy (PSPT) at a dose range of 60-74.1 Gy with concurrent chemotherapy.36 At a median follow-up of 4.7 years, median overall survival for stage II disease was 40.4 months and 30.4 months for stage III disease. Five-year disease-free rates were 17.3% and 18%, respectively.
In an analysis comparing toxicities associated with proton therapy plus chemotherapy (n = 62, median dose 74 Gy) vs. case-matched controls treated with 3D-CRT plus chemotherapy (n = 74) and IMRT plus chemotherapy (n = 66) (median dose 63 Gy for photon patients), the rates of grade ≥ 3 pneumonitis were 2% for protons, 30% for 3D-CRT, and 9% for IMRT.37 Rates of grade ≥ 3 esophagitis were 5% for protons, 18% for 3D-CRT, and 44% for IMRT. This report suggests that chemoradiation-related toxicities can be reduced with the use of protons to treat locally advanced NSCLC.
A retrospective study was published by Nakayama et al38 in which 35 patients with stage II-III NSCLC who were inoperable or ineligible for chemotherapy were treated with proton therapy to a median dose of 78.3 Gy at 2 Gy/fraction. Local progression-free survival at 1 year was 93.3% and at 2 years was 65.9%. Overall progression-free survival was 59.6% at 1 year and 29.2% at 2 years. Overall survival was 81.8% at 1 year and 58.9 at 2 years. There were no grade ≥ 3 toxicities. A second retrospective study from Japan reported on outcomes for 57 patients with stage III NSCLC treated with protons who were not able to receive chemotherapy due to age or medical comorbidities.39 The median dose was 74 Gy. Median overall survival was 21.3 months, with 1- and 2-year local control rates of 79.1% and 64.1%, respectively. Six patients experienced grade ≥ 3 lung toxicities (3 acute pneumonitis, 3 late dyspnea or hemoptysis), and no grade ≥ 3 esophagitis was observed.
In a recent report using the National Cancer Data Base of patients treated from 2004-2012, capturing 243,474 patients treated with photons and 348 patients treated with protons, demonstrated on multivariate analysis that nonproton therapy was associated with inferior overall survival [HR 1.21, p < 0.01], with propensity-matched analysis demonstrating 5-year overall survival of 22% vs. 16% (p = 0.025).40 Among stage II-III patients, photons were also associated with an increased risk of death as compared to protons (HR = 1.35, p < 0.01).
While the aforementioned studies generally show a benefit of protons compared to photons in LA-NSCLC, a Bayesian randomized trial presented at the 2016 American Society of Clinical Oncology Annual Meeting comparing 3DPT (PSPT) to IMRT, both with concurrent chemotherapy, demonstrated no statistically significant differences between the two modalities in a combined endpoint of grade ≥ 3 radiation pneumonitis or local recurrence.41 Of note, patients were only randomized if both PSPT and IMRT plans satisfied normal tissue constraints. Additionally, patients treated with proton therapy generally had larger tumor volumes (p = 0.071), were treated to higher radiation doses, and had larger lung volumes receiving ≥ 30 Gy. These limitations underscore the need for additional investigation into the benefits of proton therapy, and particularly of pencil-beam scanning proton therapy (PBSPT).
Modalities of Proton Delivery
Two main modalities deliver protons: passive scattering proton therapy (PSPT) and PBSPT.42 PSPT utilizes 3D planning, delivering a conformal dose to the tumor volume. In PSPT, scatterers are used, which reduce energy loss to ensure a uniform dose, and range modulation wheels create a spread-out Bragg peak to cover a tumor with a larger volume. PSPT is simpler to plan but it is not as precise as PBSPT. If the tumor has an irregular shape, the thinner region will receive excessive dose compared to the thicker region, as the spread-out width must be the same. And while dose is able to conform to the distal portion of the tumor volume, conformity with PSPT proximally is more limited. Also, protons stopped by scatterers create neutrons, resulting in elevated integral dose, possibly leading to long-term toxicities such as secondary malignancies.43,44
In PBSPT, computer-guided magnets are used to direct the beam, painting the tumor voxel by voxel. This is a more precise technique and is, therefore, better suited for tumors with irregular shapes. With PBSPT, one can utilize either a single-field uniform optimization (SFUD), or multiple fields optimization (MFO) to create intensity-modulated proton therapy (IMPT).45 Both SFUD and MFO use an objective function to modulate the intensities and energies of the pencil beams, delivering a targeted dose to the tumor volume while accounting for dose constraints of nearby critical structures.46 Beam-specific planning target volume based on 4D CT can be used to ensure target margin in SFUD, and robust planning must be used to ensure target coverage in MFO.47,48 The primary challenge of using IMPT for thoracic malignancies is overcoming the issue of respiratory motion. Due to the inhomogeneous beam with IMPT, tumor motion can result in regions of under-treatment or over-treatment, leading to the so-called interplay effect within treatment targets. However, various modalities and techniques (repainting, gating, fractionation, etc.) may correct for the uncertainty that results from intrafractional motion.46
Several published dosimetric studies demonstrate the benefits of IMPT.18,49 Zhang et al50 published a study that compared IMPT to PSPT and IMRT for inoperable stage IIIB disease. The plans of 10 patients who received 60-63 Gy with IMRT and 10 patients who received 74 Gy with PSPT were replanned using IMPT. Compared with both IMRT and PSPT, IMPT reduced dose delivered to uninvolved lungs and surrounding critical structures. Additionally, IMPT allowed for a dose escalation to 88.4 Gy without increasing the dose to the surrounding critical structures. The authors found that in the PSPT-treated patients, some of the plans required sacrificing part of the PTV due to dose constraints, and some of those patients had local failures.
As IMPT is relatively new, there are little published clinical data. One prospective study by Chang et al46 assessed the challenges of motion analysis and management and plan optimization in treating thoracic malignancies with IMPT. IMPT was chosen for the 34 patients in that study, as these were cases of re-irradiation or that IMPT improved dose constraints over PSPT and IMRT plans. At a median follow-up of 6.5 months for these high-risk patients, 18% of patients developed grade 2 or 3 esophagitis and 15% developed grade 2 or 3 dyspnea.
Discussion
Due to the proximity to critical structures and surrounding healthy lung tissue, treating lung cancer with radiation therapy can be challenging. Owing to their Bragg peak, protons can allow for targeted delivery of dose to lung tumors with minimal dose to surrounding tissues. With robust data demonstrating that if dose constraints can be met and toxicities can be minimized, dose escalation and hypofractionation improve local control and survival in patients with early stage NSCLC—and these interventions are continuing to be investigated in LA-NSCLC—the role of proton therapy might expand as protons may more safely allow for dose escalation and/or hypofractionation. With this and a potential toxicity reduction, protons may prove to be a cost-effective treatment modalities for thoracic tumors.51
As precise as protons are, this precision introduces challenges in treating lung cancer. As protons demonstrate steep dose gradients, intrafractional tumor motion can result in underdosing of tumor or overdosing of organs at risk. Breath hold, gating, or other motion-mitigation techniques or intrafractional tracking along with improved immobilization may be necessary when delivering proton therapy. Image-guided therapy is also vital for proton therapy implementation, and should undergo repeat 4D verification simulations during treatment to evaluate for anatomical and tumor motion changes that may occur during treatment and necessitate adaptive replanning.52-53
In comparison with IMRT planning, both the conversion of CBCT to virtual CT and the conversion of CT Hounsfield Unit to stopping power in treatment planning systems can result in the need for additional treatment margin along the proton beam direction.52 Furthermore, although in-room CBCT/CT can be used to minimize the treatment margin perpendicular to the direction of the proton beam, additional treatment margin along the beam direction is needed to account for the proton range uncertainty related to residual patient setup inaccuracies.54 To account for specific uncertainties related to organ motion and patient setup with proton therapy, which are often of a greater magnitude of importance compared to photon-based planning, as well as uncertainties with CT images conversion, robust and 4D optimization are emerging in treatment planning systems to enable the full capacity of IMPT.55-56
Future Directions
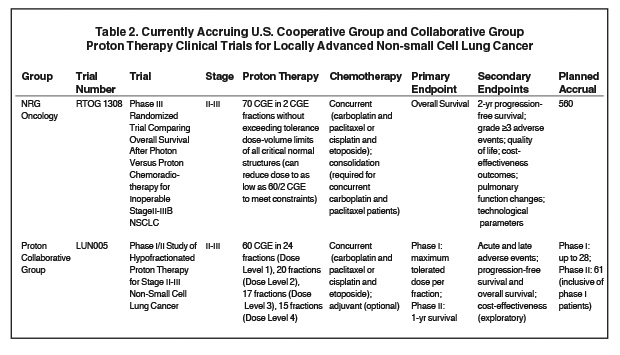
Results from prospective clinical trials are needed to be able to definitively assess for a superiority of protons compared to photons, and to identify patients most likely to benefit. RTOG 1308 is an ongoing phase III randomized trial comparing overall survival after image-guided 3D-CRT and IMRT vs. PSPT for inoperable stage II-III disease (Table 2). Patients are being treated up to 70 Gy (2 Gy per fraction), with the total dose reduced to as low as 60 Gy if dose constraints cannot be met. Patients in both arms will be treated with concurrent platinum-based chemotherapy, and secondary endpoints include progression-free survival, grade ≥ 3 adverse events, quality of life, cost-effectiveness outcomes, and pulmonary function testing changes.57-58
Another ongoing trial is Proton Collaborative Group LUN005, a phase I/II study of hypofractionated proton therapy for stage II-III NSCLC assessing the maximum tolerable dose per fraction, disease control, and toxicities/adverse events for hypofractionated proton therapy with concurrent chemotherapy.58
Most of the published studies utilize PSPT. PBSPT is a newer technology that employs small diameter beams to paint the tumor while taking dose constraints of nearby critical structures into account. Dosimetric studies have demonstrated the superiority of PBS/IMPT and early clinical data demonstrate it is safe and effective. As the number of centers that utilize PBS/IMPT grows, we will hopefully see more published data in the coming years.
References
- American Cancer Society. Cancer Facts and Figures. American Cancer Society. Atlanta, 2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed October 1, 2016.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016.
- Groome PA, Bolejack V, Crowley JJ, et al. IASLC International Staging Committee; Cancer Research and Biostatistics; Observers to the Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):694-705.
- ong F, Zhao J, Wang J, et al. Radiation dose effect in locally advanced non-small cell lung cancer. J Thorac Dis. 2014;6(4):336-347.
- Simone CB, Wildt B, Haas AR, et al. Stereotactic body radiation therapy for lung cancer. Chest. 2013 Jun 1;143(6):1784-1790.
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small cell lung cancer: a phase III randomised control trial. Lancet. 2009;374:379-386.
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;2(16):187-199.
- Levin WP, Kooy H, Loeffler JS, et al. Proton beam therapy. Br J Cancer. 2005;93(8):849-854.
- Roelofs E, Engelsman M, Rasch C, et al. ROCOCO Consortium. Results of a multicentric in silico trial (ROCOCO): comparing radiotherapy with photons and protons for non-small cell lung cancer. J Thorac Oncol. 2012;7:165-176.
- Socinski MA, Morris DE, Halle JS, et al. Induction of concurrent chemotherapy and high-dose thoracic conformal radiation therpay in unresectable stage IIIA and IIIB non-small cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004;22:4341-4350.
- Simone CB 2nd, Rengan R. The use of proton therapy in the treatment of lung cancers. Cancer J. 2014;20(6):427-432.
- Stephans K. Stereotactic body radiotherapy for stage I non-small cell lung cancer. Cleve Clin J Med. 2012;79:eS26-31.
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7):S94-S100.
- Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med. 2015;3(13):172.
- Segawa Y, Takigawa N, Kataoka M, et al. Risk factors for development of radiation pneumonitis following radiation therapy with or without chemotherpay for lung cancer. Int J Radiat Oncol Biol Phys. 1997;39:91-98.
- Song SY, Choi W, Shin SS, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer. 2009;66:89-93.
- Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. J Thorac Oncol. 2016;11(7):1081-1089.
- Register SP, Zhang X, Mohan R, et al. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage i non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;80(4):1015-1022.
- Macdonald OK, Kruse JJ, Miller JM, et al. Proton beam radiotherapy versus three dimensional conformal stereotactic body radiotherapy in primary peripheral, early-stage non-small cell lung carcinoma: a comparative dosimetric analysis. Int J Radiat Oncol Biol Phys. 2009;75:950-958.
- Wang C, Nakayama H, Sugahara S, et al. Comparisons of dose-volume histograms for proton-beam versus 3-D Conformal x-ray therapy in patients with stage I non-small cell lung cancer. Strahlenther Onkol. 2009; 185(4):231-234.
- Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early stage lung cancer. Chest. 1999;116:1313-1319.
- Bush DA, Slater JD, Shin BB, et al. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest. 2004;126:1198-1203.
- Bush DA, Cheek G, Zaheer S, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective. Int J Radiat Oncol Biol Phys. 2013;86:964-968.
- Shioyama Y, Tokuuye K, Okumura T, et al. Clinical evaluation of proton radiotherpay for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;56:7-13.
- Hata M, Tokuuye K, Kagei K, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys. 2007;68:786-793.
- Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the University of Tsukuba. Int J Radiat Oncol Biol Phys. 2010;78:467-471.
- Kanemoto A, Okumura T, Ishikawa H, et al. Outcomes and prognostic factors for recurrence after high-dose proton beam therapy for centrally and peripherally located stage I non-small cell lung cancer. Clin Lung Cancer. 2014;15:e7-e12.
- Nihei K, Ogino T, Nishimura H. High-dose proton beam therapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(1):107-111.
- Iwata H, Murakami M, Demizu Y, et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer. 2010;116:2476-2485.
- Iwata H, Demizu Y, Fujii O, et al. Long-term outcome of proton therapy and carbon-ion therapy for large (T2a-T2bN0M0) non-small-cell lung cancer. J Thorac Oncol. 2013;8:726-735.
- Makita C, Nakamura T, Takada A, et al. High-dose proton beam therapy for stage I non-small cell lung cancer: clinical outcomes and prognostic factors. Acta Oncologica. 2015;54(3):307-314.
- Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J radiat Oncol Biol Phys. 2006;65:1087-1096.
- Chang J, Komaki R, Lu C , et al. Phase II study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III non-small cell lung cancer. Cancer. 2011;117:4707-4713.
- Bradley JD, Bae K, Graham MV, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol. 2010;28(14):2475-2480.
- Xiang ZL, Erasmus J, Komaki R, et al. FDG uptake correlates with re-recurrence and survival after treatment of unresectable stage III non-small cell lung cancer with high-dose proton therapy and chemotherapy. Radiat Oncol. 2012;7:144.
- Nguyen Q, Ly N, Komaki R, et al. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol. 2015;115(3):367-372.
- Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117(13):3004-3013.
- Nakayama H, Satoh H, Sugahara S, et al. Proton beam therapy of stage II and III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:979-984.
- Oshiro Y, Mizumoto M, Okumura T, et al. Results of proton beam therapy without concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2012;7:370-375.
- Higgins KA, O’Connell K, Liu Y, et al. National Cancer DataBase Analysis of Proton versus Photon Radiotherapy in Non-Small Cell Lung Cancer (NSCLC). Int J radiat Oncol Biol Phys. 2016; doi: 10.1016/j.ijrobp.2016.10.001.
- Liao Z, Lee J, Komaki R, et al. Bayesian randomized trial comparing intensity modulated radiation therapy versus passively scattered proton therapy for locally advanced non-small cell lung cancer. J Clin Oncol. 2016; abstract 8500.
- Wink KC, Roelofs E, Solberg T, et al. Particle therapy for non-small cell lung tumors: where do we stand? A systematic review of the literature. Front Oncol. 2014;4(292).
- Liu H, Chang JY. Proton therapy in clinical practice. Chin J Cancer. 2011;30(5).
- Simone CB 2nd, Kramer K, O’Meara WP, et al. Predicted rates of secondary malignancies from proton versus photon radiation therapy for stage I seminoma. Int J Radiat Oncol Biol Phys. 2012;82(1):242-249.
- Chang JY, Jabbour SK, De Ruysscher D, et al. Consensus statement on proton therapy in early stage and locally advanced non-small cell lung cancer. Int J Radiation Oncol Biol Phys. 2016;95(1):505-516.
- Chang JY, Heng L, Zhu X, et al. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys. 2014;90(4):809-818.
- Lin L, Kang M, Solberg TD, et al. Use of a novel two-dimensional ionization chamber array for pencil beam scanning proton therapy beam quality assurance. J Appl Clin Med Phys. 2015;16(3):270-276.
- Lin L, Kang M, Huang S, et al. Beam-specific planning target volumes incorporating 4D CT for pencil beam scanning proton therapy of thoracic tumors. J Appl Clin Med Phys. 2015;16(6):5678.
- Kesarwala A, Ko C, Ning H, et al. Intensity-modulated proton therapy for elective nodal irradiation and involved-field radiation in the definitive treatment of locally advanced non-small-cell lung cancer: a dosimetric study. Clin Lung Cancer. 2015;16:237-244.
- Zhang X, Li Y, Pan X, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer. Int J Radiat Oncol. 2010;77(2):357-366.
- Verma V, Shah C, Rwigema JC, et al. Cost-comparativeness of proton versus photon therapy. Chin Clin Oncol. 2016;5(4):56.
- Veiga C, Janssens G, Teng CL, et al. First clinical investigation of cone beam computed tomography and deformable registration for adaptive proton therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):549-559.
- Koay EJ, Lege D, Mohan R, et al. Adaptive/nonadaptive proton radiation planning and outcomes in a phase II trial for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;84(5):1093-1100.
- Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: The potential effects of calculational uncertainties. Phys Med Biol. 2008;53(4):1027-1042.
- Yu J, Zhang X, Liao L, et al. Motion-robust intensity-modulated proton therapy for distal esophageal cancer. Med Phys. 2016;43(3):1111-1118.
- Liu W, Schild SE, Chang JY, et al. Exploratory study of 4D versus 3D robust optimization in intensity modulated proton therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):523-533.
- Giaddui T, Chen W, Yu J, et al. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: a phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol. 2016;11(16).
- ClinicalTrials.gov, “Search for Clinical Trials.” Available at: http://www.clinicaltrials.gov/. Accessed October 1, 2016.
Citation
L CJL, SJ F, II SC. Proton therapy for lung cancer: Current uses and future applications for early stage and locally advanced non-small cell lung ca. Appl Radiat Oncol. 2016;(4):12-18.
December 12, 2016