Prevalence of Pediatric Advanced Life Support Training Among Radiation Oncology Residency Programs
Images


Abstract
Purpose: Cardiac arrest is a recognized complication of pediatric oncology management, and timely and correct administration of Pediatric Advanced Life Support (PALS) improves return of spontaneous circulation (ROSC).
Methods and Materials: We designed a 6-item internet-based survey, distributed to the 92 program coordinators at US radiation oncology residency programs, which assessed the prevalence of PALS training and potential associated factors among residents. Ordinal and categorical variables were obtained; tests for association with the PALS requirement included Fisher’s exact and Spearman’s rank correlation.
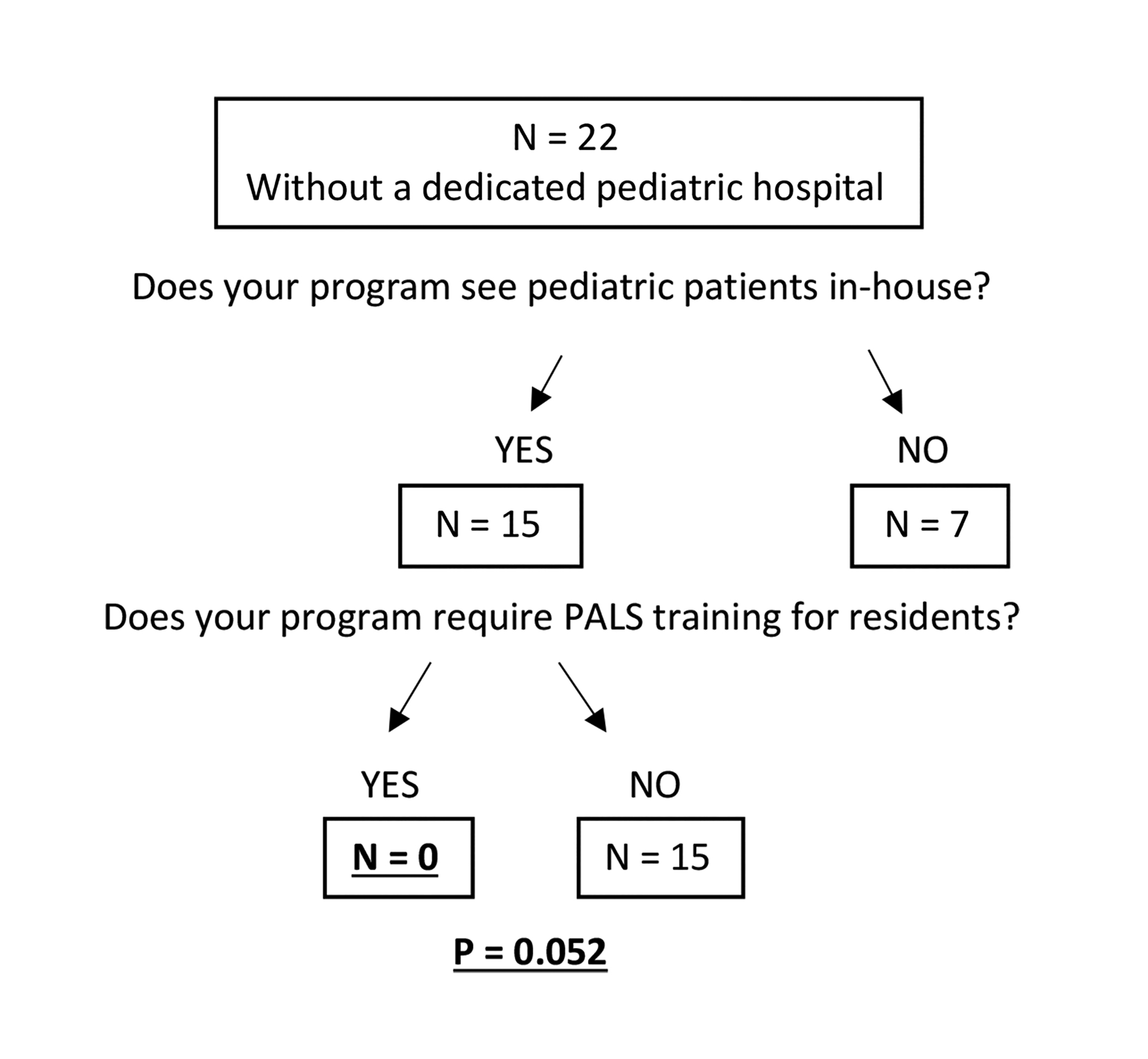
Results: Sixty-two of 92 residency programs responded in full (67.4%). PALS training is required at 11 of 62 programs (17.7%). Fifty of 62 programs see pediatric patients in-house (80.6%); 38 of these 50 programs also utilize away sites. Forty of 62 programs (64.5%) are associated with a dedicated pediatric hospital. The most common number of residents per program is 7 to 10 (38.7% of programs). Residency programs most commonly (38.7%) have residents focus on pediatric cases for at least 4 months. Most commonly, residents see 12 to 16 pediatric cases over their 4-year training period (40.3% of programs). Of the 15 programs that see pediatric cases intradepartmentally and are not affiliated with a dedicated pediatric hospital, none require PALS training (P = 0.052). Neither the size of the residency program, number of months focused on pediatric cases, nor number of total pediatric cases seen by residents over the 4-year training period is significantly associated with the requirement of PALS certification.
Conclusion: Despite the preponderance of intradepartmental pediatric visits and the presence of on-site pediatric hospitals, results suggest that fewer than 1 in 5 US radiation oncology residency programs require PALS certification. Given radiation oncology residents’ significant exposure to ill children, we recommend that the requirement for PALS certification during training be reconsidered.
Each year in the United States, an estimated 15,000 pediatric hospital patients experience cardiac arrest and require cardiopulmonary resuscitation (CPR).1 The CPR algorithm designated for the peri-arrest management of children was designed in 1983 by the American Heart Association (AHA), and integrated into the first Pediatric Advanced Life Support (PALS) courses in 1988.2 Most recent updates to PALS guidelines were published by the AHA in 2018, alongside corresponding updates to its Advanced Cardiovascular Life Support (ACLS) program designed for adults. Fundamental distinctions in life support delivery are highlighted in these guidelines, reinforcing that delaying proper PALS administration lowers the incidence of return of spontaneous circulation (ROSC).3
While ACLS is generally regarded as a prerequisite for radiation oncology credentialing in American hospital systems, PALS is not. This shortcoming is concerning given that pediatric radiation oncology patients are typically seen in a clinic that is separate from designated pediatric staff. We hypothesized that despite the significant pediatric population among radiation oncology patients, as well as the nationally prescribed requirement of a minimum of 12 pediatric cases for graduation, PALS training is rarely required within radiation oncology residency programs. To examine this proposition, we designed and distributed a survey that assesses the prevalence of PALS training at radiation oncology residency programs, as well as potential factors associated with programs requiring PALS training for residents in the United States.
Methods and Materials
Survey Instrument
We designed an internet-based survey, managed and distributed by Qualtrics, which consisted of 6 multiple choice questions designed to be completed within 5 minutes (See Supplement). The questions assessed the requirement for radiation oncology resident PALS training, number of residents in the program, total number of pediatric patients, total months specifically focusing on pediatric cases, whether the department is associated with a dedicated pediatric hospital, and whether pediatric patients are seen by residents intradepartmentally or at an away site.
The Columbia University Irving Medical Center Institutional Review Board (IRB) reviewed our application and determined our project did not qualify as human subjects research; therefore, IRB-approval was granted yet not required.
Survey Procedure
Radiation oncology residencies and their associated program coordinators (PC) were identified through the American Council for Graduate Medical Education (ACGME) public search for accredited American programs as of October 2019. Beginning November 2019, each PC was contacted via his/her work email address and provided a link to a Qualtrics-based online survey. There were no exclusion criteria. All email correspondences to potential participants consisted of non-individualized form letters and were distributed by the residency program coordinator at the authors’ institution. Approximately every 8 weeks, the same original recipients were sent a reminder to participate in the survey if not yet completed; this reminder email was sent twice over 4 months. No other intervention was undertaken to improve the response rate.
Statistical Analysis
Data were analyzed with SPSS version 26.0 (IMB Corp). Responses were compiled as categorical or ordinal variables. In lieu of numerical variables, the survey grouped quantitative data into groups for ordinal analysis. Tests for association with PALS training included Fisher’s exact test with Freeman-Halton extension and Spearman’s rank correlation. Statistical significance was two-tailed and defined as a P-value < 0.05.
Results
The survey accrued responses from November 2019 through March 2020. Replies were received from 66 of 92 programs surveyed (71.4%); however, 4 of these contained no responses past the consent page. Therefore, this study examines 62 of 92 analyzable surveys (67.4%). PALS training is required at 11 of 62 residency programs (17.7%). Fifty of 62 programs see pediatric patients in-house (80.6%), with 38.0% of these 50 programs also utilizing away sites. Forty of 62 programs (64.5%) are associated with a dedicated pediatric hospital.
Twenty-four of 62 programs (38.7%) have 7 to10 residents, 19 of 62 programs (30.6%) have 4 to 6 residents, and the remainder have 11-plus residents. (30.6%). Twenty-four of 62 programs (38.7%) have residents focus on pediatric cases for 4-plus months, 29.0% for 3 months, 12.9% for 2 months, 14.5% for 1 month, and 4.8% for 0 months. Twenty-five of 62 programs (40.3%) have residents see 12 to 16 pediatric cases over their 4-year training period, 16.1% each for 17 to 21 or 22 to 26 cases, 11.3% for < 12 cases, and 8.1% each for 27 to 31 or 32-plus cases (Figure 1).
A requirement of PALS training among residents is not associated with residents seeing pediatric patients at their home institution (or both home and away) vs away sites only (P = 0.907), nor with presence of a dedicated pediatric hospital (P = 0.300). No association with PALS was seen with number of residents within the program (rs = 0, P = 1.00), number of months focused on pediatric cases (rs = 0.088, P = 0.498), or number of total pediatric cases (rs = 0.166, P = 0.198).
In the subgroup of 15 residency programs (24.2% of cohort) that both saw patients in-house yet were not affiliated with a dedicated pediatric hospital, none of the 15 programs required PALS training (P = 0.052) (Figure 2). PALS training was additionally not associated with the combination of in-house pediatric visits plus the presence of a pediatric hospital (35 of 62 programs (56.4%), P = 0.094), nor was the number of pediatric cases seen by this subgroup shown to be correlated with PALS training (rs = 0.259, P = 0.133).
Discussion
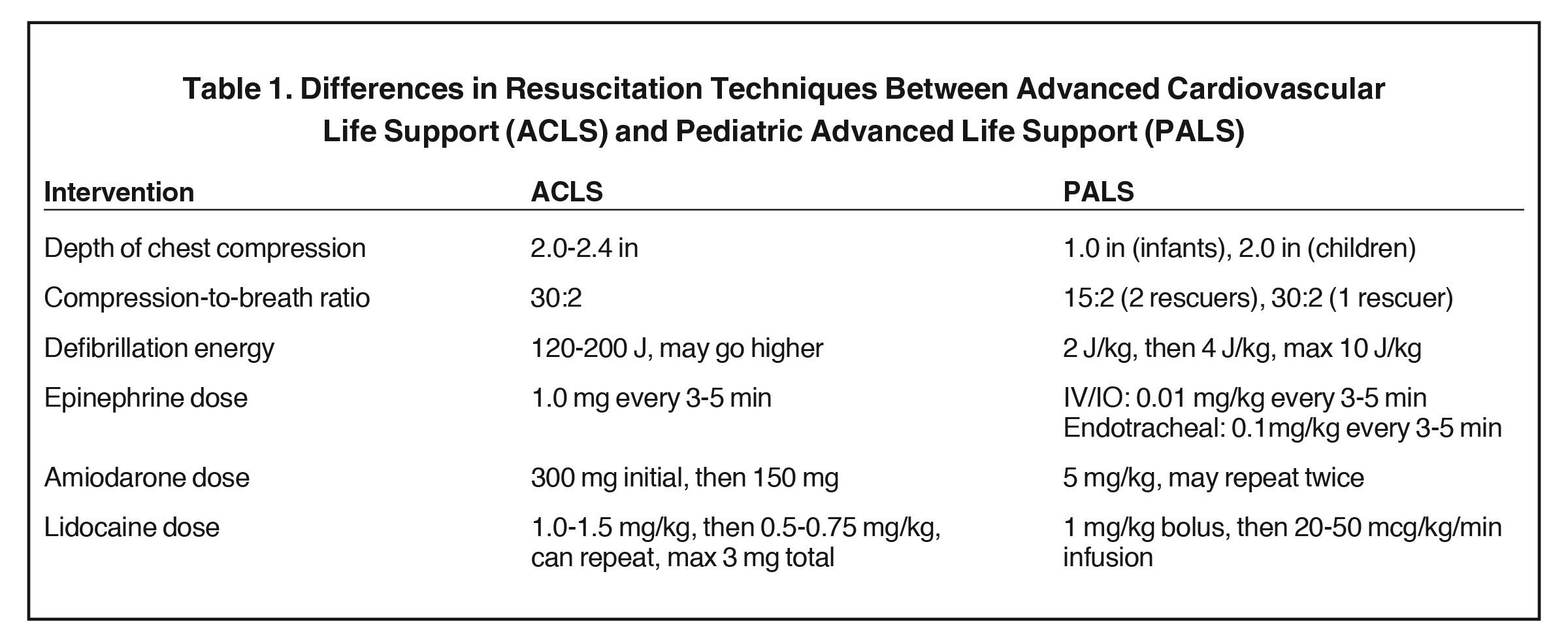
Current evidence-based recommendations highlight fundamental distinctions in cardiac arrest management between children and adults. Owing to intrinsic differences in anatomy and mass, adjustments in chest compression techniques are required for children, as well as energy reductions in defibrillation shocks and dose reductions in systemic sympathetic agonists such as epinephrine and antiarrhythmics such as amiodarone.4 Delay in proper administration of PALS leads to a lower incidence of the return of spontaneous circulation (ROSC)3 (Table 1).
For multiple reasons, cancer patients are at increased risk for cardiac arrest as compared to the general population. The hypercoagulable state associated with malignancy significantly increases the risk of thromboembolic events, and exposure to nephro-, hepato-, and cardiotoxic chemotherapies, additionally, are associated with higher incidence of cardiac arrest.5 Chronic kidney disease precipitates fibrosis and coronary artery calcification and, thus, is an independent risk factor for cardiac death. As a population, cancer patients have worsened kidney function due to exposure to nephrotoxic chemotherapeutic agents.6 Additionally, use of anesthesia, which is required for pediatric patients far more frequently than for adult patients receiving radiation therapy, is associated with respiratory arrest that requires management with PALS or ACLS algorithms. A recent study showed that nearly 20% of anesthesia-related pediatric cardiac arrests occurred in the emergence or recovery period; these arrests occurred despite the patients being within a dedicated post anesthesia care unit (PACU) setting.7 While the total number of arrests was low, this study blamed inadequate supervision and provider inexperience. Many radiation oncology patients recover from anesthesia in a clinic setting vs a dedicated PACU, with the final stages of recovery occurring after the anesthesiologist and support technicians have departed. Further research into the logistics of pediatric anesthesia in radiation oncology is needed to identify best practices regarding induction and recovery locations.
To our knowledge, this project is the first survey to assess the prevalence of the requirement for PALS training at radiation oncology residencies throughout the United States. The results suggest that < 1 in 5 American radiation oncology residency programs requires PALS certification for resident physicians. This low representation exists despite the preponderance of intradepartmental pediatric visits and the presence (or lack) of on-site pediatric hospitals.
Our results highlight a particularly dangerous situation in programs where pediatric cancer patients are seen intradepartmentally and yet without support of a dedicated pediatric hospital. Of the 15 such programs, none required PALS training; this finding approached statistical significance (P = 0.052) despite the small sample size.
We hypothesized that programs seeing greater numbers of pediatric patients or that have greater numbers of residents would be more likely to require PALS; however, our findings suggest that a requirement for resident training in PALS is not associated with greater numbers of pediatric cases, months dedicated to pediatric patients, or number of residents in a residency program.
In the era of COVID-19, with potentially reduced staff and with multiple reports of catastrophic cardiac events in critically ill children, and especially given the short period required for PALS training and its potential benefit to improve the rate of ROSC, training of radiation oncology residents in PALS may represent a high-value quality improvement initiative.8 Given the team-based nature of PALS and other advanced life support algorithms, training and proficiency in multiple members of the care team contribute to optimal outcomes. Therefore, while this survey did not assess the prevalence of PALS certification among other members of the radiation oncology care team such as attending physicians and nurses, the fact that radiation oncology residents are routinely trained in ACLS but not PALS identifies a training domain that may improve outcomes for patients. Further, in due time many of these residents will give rise to new attending physicians who are already PALS-certified. Ultimately, the question of who makes the policy decision toward or against implementing PALS training in a radiation oncology program, and for what reasons, is beyond the scope of our survey.
The strength of this small study is the strong response rate of nearly 70%. Curiously, however, 7 programs (11.3%) indicated that their residents see < 12 pediatric patients during their residency; nationally prescribed residency guidelines mandate a minimum of 12 patients for graduation. This dilemma might call into question the validity of their responses. A limitation of our study is that the absolute risk reduction for incorporating PALS training into radiation oncology residency programs is unknown.
Conclusion
Few radiation oncology residency programs require PALS training. Given radiation oncology residents’ significant exposure to ill children at elevated risk of cardiopulmonary arrest, radiation oncology residency program directors and graduate medical education leadership should evaluate implementation of PALS training among radiation oncology residents.
References
- Holmberg MJ, Ross CE, Fitzmaurice GM, et al. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12(7):e005580.
- Mutchner L. The ABCs of CPR--again. Am J Nurs. 2007;107(1):60-69;quiz 69-70.
- Andersen LW, Berg KM, Saindon BZ, et al. Time to epinephrine and survival after pediatric in-hospital cardiac arrest. J Am Med Assoc. 2015;314(8):802-810.
- Craig-Brangan KJ, Day MP. Update: 2017/2018 AHA BLS, ACLS, and PALS guidelines. Nursing. 2019;49(2):46-49.
- Sardar M, Shaikh N, Malik SU, et al., Possible predictive factors for in-hospital cardiac arrest in patients with cancer: a retrospective single center study. Cureus. 2018;10(6):e2828.
- Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol. 2012;23(12):929-939.
- Christensen RE, Haydar B, Voepel-Lewis TD. Pediatric cardiopulmonary arrest in the postanesthesia care unit, rare but preventable: analysis of data from wake up safe, the Pediatric Anesthesia Quality Improvement Initiative. Anesth Analg. 2017;124(4): 231-1236.
- Belhadjer Z, Meot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 May 17.
Citation
Garrett, KA S, M R, J T, LA K, CC W, DP H. Prevalence of Pediatric Advanced Life Support Training Among Radiation Oncology Residency Programs. Appl Radiat Oncol. 2020;(4):26-30.
December 24, 2020