Postprostatectomy radiation therapy for biochemically recurrent prostate cancer
Images

Abstract
Objective: This series retrospectively reviewed the treatment strategy of salvage radiation therapy for patients for whom prostate-specific antigen (PSA) has already demonstrated failure after a period of observation following prostatectomy.
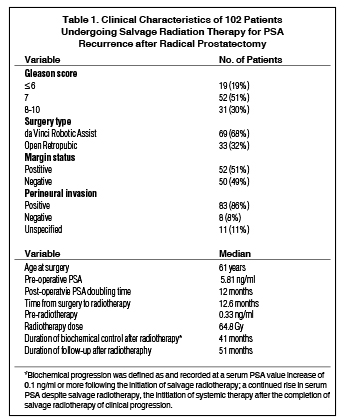
Methods and Materials: At our institution, 102 patients were treated with salvage radiation therapy, 19 of whom had a Gleason score ≤ 6, 52 of whom had Gleason 7, and 31 of whom had Gleason ≥ 8 prostate cancers. Median follow-up after radiation therapy was 51 months. The median PSA prior to salvage radiation therapy was 0.33, and the median time from prostatectomy to radiation therapy was 24.6 months. Positive margins were identified in 52 patients, and perineural invasion was positive in 83. The median dose delivered was 64.8 Gy.
Results: The 5-year actuarial freedom from biochemical failure rates for National Comprehensive Cancer Network (NCCN) low-, intermediate-, and high-risk groups were 100%, 77%, and 62%, respectively (p = 0.2449). The 5-year actuarial freedom from biochemical failure rates for a Gleason score ≤ 6, Gleason 7, and Gleason ≥ 8 patients were 87%, 72%, and 49%, respectively (p = 0.0187). Patients with pre-radiation therapy PSA ≤ 0.5 had better 5-year biochemical control relative to patients with higher pre-radiation therapy PSA, 76% vs. 51% (p = 0.0211). Few interval biochemical failures are observed after the 5-year point of follow-up. The 5-year overall survival for the entire cohort is 92%, with prostate-cancer-specific survival of 96%.
Conclusions: Salvage radiation therapy demonstrated durable PSA control and few failures at 5 years post-radiation. Initiation of salvage radiation therapy for PSA ≤ 0.5 demonstrated improved biochemical control, supporting the adoption of early referral to radiation oncology once post-prostatectomy biochemical failure is identified.
Approximately one-third of affected men choose to undergo radical prostatectomy as definitive therapy for prostate cancer,1 and roughly 15% to 35% of these men will experience biochemical recurrence of prostate cancer within 10 years, which is denoted by an increase in serum prostate-specific antigen (PSA).2-4 It is generally accepted that salvage radiation therapy (SRT), defined as the initiation of radiation therapy upon the identification of biochemical recurrence, offers the best prognosis for patients without distant metastases. Adjuvant radiation therapy (ART), another treatment technique commonly used for patients exhibiting adverse pathological features (APF) at the time of surgical prostate resection, employs radiation therapy as an immediate adjunct to surgical resection. The use of adjuvant vs. salvage radiation therapy is the subject of ongoing randomized trials. Current guidelines recommend that patients exhibiting adverse pathology indicating a high risk of recurrence should be offered ART.5 Although ART has been shown to be effective in certain patients, risks may outweigh benefits in others. Therefore, employing ART as the standard of care would expose some patients to unnecessary doses of radiation.6
Our series reviews the common treatment strategy of salvage radiation therapy for patients in whom serum PSA values have demonstrated biochemical recurrence after a period of observation following prostatectomy. Salvage radiation therapy represents a curative treatment option for patients who exhibit biochemical failure following prostate resection.7 The primary goal of this study was to explore our institutional experience and use it to determine if initiating SRT before a specific serum PSA marker value led to better patient outcomes in our cohort. Currently, no official consensus definitively declares the optimal serum PSA cutoff value at which SRT should be initiated. Here we present a retrospective analysis of 102 consecutive patients treated with postprostatectomy salvage radiation therapy.
Materials and Methods
Participants
Between March 2003 and June 2014, 102 patients underwent salvage radiation therapy at a community hospital after biochemical recurrence of localized prostatic adenocarcinoma following radical prostatectomy. All patients were treated with curative intent by multiple physicians following the same departmental protocol. National Comprehensive Cancer Network (NCCN) risk stratification was used to predict the probability of postprostatectomy biochemical failure. Four patients were classified as low risk, 37 as intermediate risk, and 61 as high risk based on PSA values and histopathological features. Face-to-face follow-up with PSA testing and digital rectal exam after radical prostatectomy took place in the office setting and varied based on physician preference. Identifying patient information was stripped by the cancer registrar. The study was reviewed and approved by the institutional review board.
Inclusion and Exclusion
Patients included in the study underwent radical prostatectomy for prostatic adenocarcinoma, developed subsequent biochemical PSA failure and were treated with SRT at our facility. Ultrasensitive PSA assays were used to detect increased serum PSA values indicating biochemical recurrence before deciding whether to initiate SRT. Subjects were chosen based on site (prostate), histology (Gleason score and tumor node metastasis [TNM] staging), surgical resection (prostatectomy), recurrence (biochemical failure) and postrecurrence treatment (radiation). Exclusion criteria were stage T4 cancer, any radiation not done at our facility, any patient who underwent chemotherapy, and any patient with a secondary active cancer other than prostatic adenocarcinoma. Patients with a history of a secondary cancer type that was either inactive or in a period of follow-up after radiation therapy were not excluded from the study.
Treatment
Patients were staged with a bone scan and computed tomography (CT) of the abdomen and pelvis to ensure no distant metastatic disease prior to treatment. The prostate fossa clinical target volume (CTV) was defined to include the posterior bladder and residual seminal vesicles superiorly down to the vesicourethral anastomosis inferiorly, including the urogenital diaphragm. The contents anterior to the rectum and posterior to the pubic symphysis were targeted with a margin for setup uncertainty of 0.7 to 1 cm in all directions, except posteriorly where the margin was 0.5 to 0.7 cm. The elective treatment of the pelvic lymph node basins was left to the discretion of the treating physician, and 73 patients had elective nodal radiation followed by a boost to the prostatic fossa. Three-dimensional conformal radiation therapy (3DCRT) was delivered for 10 patients and intensity-modulated radiation therapy (IMRT) was delivered for 92 patients. The dose delivered to each patient was within a range of 58 Gy to 75 Gy, with a median dose of 64.8 Gy. Fourteen patients received adjuvant androgen deprivation therapy (ADT) with a gonadotropin-releasing hormone (GnRH) agonist and/or anti-androgen agent upon completing salvage radiation therapy, while 88 did not. Optimal treatment strategy with ADT was determined according to physician preference.
Outcomes
Five-year actuarial freedom from biochemical failure was the primary outcome evaluated in this study. Secondary outcomes were overall patient survival and prostate-cancer-specific survival. Biochemical progression indicating failure was defined as and recorded at a serum PSA value of 0.1 ng/mL or more following the initial SRT, a continued rise in serum PSA despite continued SRT, the initiation of systemic therapy after the completion of SRT, or clinical progression.
Statistical Analysis
Actuarial freedom from biochemical progression was calculated using the Kaplan-Meier method for the entire cohort and with respect to prognostic variables. Estimated survival curves for patient subgroups were compared by utilizing the log-rank test to calculate statistical significance, which was evaluated at the conventional significance level of 0.05 for all considerations. Statistical analyses were performed using MedCalc statistical software (MedCalc Software, Belgium).
Results
Specific patient characteristics are detailed in Table 1. The average and median age at the time of surgery was 61 years, with a standard deviation of ± 7. Median pre-operative PSA was 5.81 ng/mL. Only 19% of patients received a surgical Gleason score of < 6, 51% were given a score of 7, and 30% scored > 8. Pathologic stage was T2 in 48% of patients, T3a in 23%, and T3b in 24%. Perineural invasion was identified in 81% of patients and positive surgical margins were identified in 51%. Lymph node sampling was performed in 46 patients, and only 1 patient had pathological evidence of nodal involvement. Lymph node sampling was not predictive for negative surgical margins. Patients treated with open prostatectomy had a positive surgical margin rate of 52% and an overall high-grade disease rate of 21%, while patients treated with da Vinci robotic-assisted prostatectomy had a positive margin rate of 49% and overall high-grade disease rate of 34%. Median postoperative PSA doubling time (PSADT) was 12 months and the median interval from prostatectomy to the initiation of radiation therapy following biochemical recurrence was 12.6 months. Median PSA for the cohort before the initiation of salvage radiation therapy was 0.33 ng/mL.
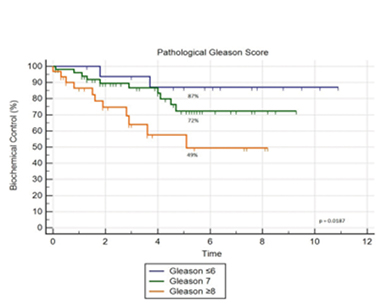
Twenty-three men in the cohort eventually experienced biochemical progression during the observational period following radiation treatment. The median age of these men was 62. As seen in Figure 1, 5-year actuarial freedom from biochemical failure rates for NCCN low, intermediate, and high-risk groups were 100%, 77%, and 62%, respectively (p = 0.2449). Statistical analysis using the log-rank test demonstrated a particularly significant association among groups in remission at 5 years based on Gleason scoring criteria. Five-year actuarial freedom from biochemical failure rates for patients with a Gleason score of ≤ 6, Gleason 7, and Gleason 8-10 were at 87%, 72%, and 49%, respectively (p = 0.0187), as illustrated in Figure 2.
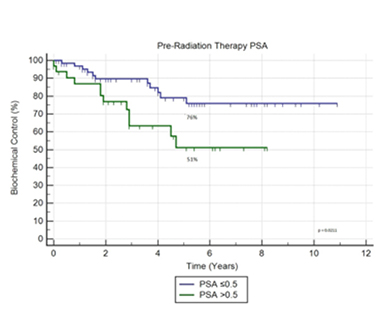
Patients with pre-radiation therapy PSA ≤ 0.5 ng/mL had better 5-year biochemical control relative to patients with higher pre-radiation therapy PSA values—76% vs. 51% (p = 0.0211)—as shown in Figure 3. Pathological margin status did not predict for biochemical control after salvage radiation across the different Gleason grades. For Gleason ≤ 6, positive margin patients vs. negative was 84% vs. 89% controlled (p = 0.8484). For Gleason 7, positive margin patients vs. negative was 71% vs. 74% controlled (p = 0.9803). For Gleason 8-10, positive margin patients vs. negative was 37% vs. 66% controlled (p = 0.4515). Pathologic T stage did not reach statistical significance (p = 0.1932), although 5-year biochemical control rates were 82% for stage T2 tumors, 67% for stage T3a tumors, and 55% for stage T3b tumors.
Subset analysis was performed to exclude the 14 patients who received ADT immediately following salvage radiation therapy. Of the 88 patients who did not receive ADT, 5-year actuarial freedom from biochemical failure rates for Gleason score ≤ 6, Gleason 7, and Gleason ≥ 8 patients were 86%, 77%, and 50%, respectively (p = 0.0347). Interestingly, withholding patients who received ADT from the analysis increased the 5-year biochemical control rate in patients with pre-radiation therapy PSA ≤ 0.5 ng/mL from 76% to 87%. Patients who received adjuvant ADT did exhibit both higher mean and median pre-radiation therapy PSA values of 1.8 ng/mL and 0.38 ng/mL, respectively, vs. a mean of 1.02 ng/mL and median of 0.33 ng/mL for the entire group.
Nine patients in the group of 102 died throughout the follow-up period and 4 of those deaths were documented as prostate cancer specific. All 4 of these patients were being treated for metastatic disease at the time. Of the 5 additional patients who died, none had experienced biochemical recurrence of localized prostate cancer following salvage radiation therapy. Five-year overall survival for the entire cohort is 92%, with prostate-cancer-specific survival of 96%. Very few interval biochemical failures are observed after the 5-year point of follow-up, as seen in the Kaplan-Meier curves, indicating durable disease control after 5 years.
Discussion
This consecutive case analysis demonstrates that salvage radiation therapy remains a curative option for patients in whom it may be undesirable to initiate adjuvant radiation. Because many patients treated with surgical prostate resection will never develop biochemical failure, avoidance of ART prevents such patients from receiving unnecessary treatment with radiation. D’Amico et al studied 1638 men who underwent radical prostatectomy and found no increased risk of all-cause mortality between groups treated with ART vs. SRT.8 Furthermore, a 16-year, 890-patient study of men staged with pT3N0 prostate cancer following surgical resection identified no significant difference in 5-year biochemical recurrence and survival rates amongst groups treated with either ART or SRT administered at PSA ≤ 0.5 ng/mL after a period of initial observation.9 This result contrasts with SWOG-S8794, which revealed that ART in men exhibiting evidence of extra-prostatic invasion on pathological sections (T3N0M0) produced a significant reduction in metastatic disease incidence with improved survival benefit.10 Interpretation of SWOG-S8794 has notably shaped the current school of thought regarding post-prostatectomy follow-up and treatment, characterized by a cautious approach to men exhibiting APF with heavy consideration of ART in this subset. A recent meta-analysis of 2629 patients suggested clinicians recommend ART to all patients displaying APF, citing increased overall and disease-free survival at 3 and 5 years.11 Radiotherapy–Adjuvant Versus Early Salvage (RAVES) is an ongoing phase III randomized, controlled clinical trial slated to run through 2021 that will further investigate the application of ART vs. SRT in patients undergoing surgical prostate resection.12 For now, multiple analyses of ART vs. SRT continue to support dissenting conclusions. Clinicians should continue to proceed with caution when treating patients displaying APF until additional studies clarify the opposing findings between these divergent treatment arms.
SRT also represents a therapeutic option for patients when ADT is undesired. Indeed, a vital point to consider is that 88 of the 102 patients reviewed in our series did not undergo ADT. A study of 635 patients with biochemical recurrence after prostatectomy at Johns Hopkins previously demonstrated that adjuvant use of ADT during salvage radiation therapy did not significantly improve outcomes.13 The freedom from biochemical failure and high survival rate of the entire cohort in our series seems to support those findings. RTOG-9601, a double-blind, placebo-controlled trial, found that 24 months of daily bicalutamide led to significantly improved overall patient survival with decreased rates of secondary metastases in patients undergoing SRT.14 However, patients with higher PSA levels prior to treatment exhibited the greatest overall survival benefit. Interestingly, in both RTOG-9601 and our institutional analysis, patients exhibited marginally improved outcomes with lower pre-treatment PSA values when receiving SRT alone in comparison to those receiving ADT in addition to SRT. Siddiqui et al found that patients who received adjuvant ADT within 90 days of surgery showed mildly improved rates of 10-year progression-free (95% vs. 90%) and cancer-specific survival (98% vs. 95%), but those who received adjuvant ADT following biochemical recurrence at PSA values between 0.4 ng/mL and 1.0 ng/mL exhibited adverse rates of 10-year progression-free survival (75% treated vs. 80% untreated) and cancer-specific survival (86% treated vs. 91% untreated).15 It is possible that with biochemical recurrence identified at lower serum PSA values prior to SRT, the additional use of ADT with certain agents can be disadvantageous. GETUG-AFU 16, another randomized, controlled multicenter trial, observed significantly improved 5-year biochemical control for patients treated with SRT plus goserelin, a GnRH agonist, when compared to SRT alone (80% vs. 62%), a consistent theme among all measured pre-trial PSA value patient subsets.16 Radiotherapy and Androgen Deprivation in Combination with Local Surgery (RADICALS), an incomplete large-scale phase III randomized, controlled clinical trial, aims to assess the various roles of ART, SRT and ADT.17 Hopefully, data gathered from RADICALS will help shed light on the indefinite role of ADT in the setting of SRT, in addition to several other dominant debates concerning existing postprostatectomy patient care.
Multivariate analysis of GETUG-AFU 16 determined that PSADT, surgical margin status, seminal vesicle status and pre-radiation therapy serum PSA values accorded no predictive value for detecting future biochemical failures.16 Among these 4 factors, we found that a pre-radiation therapy PSA marker value of 0.5 ng/mL or less was a significant factor in gauging the likelihood of 5-year biochemical control (p = 0.0211). Briganti et al revealed that patients with less favorable histopathological features following prostate resection had a significantly amplified probability of experiencing biochemical failure when pre-radiation therapy PSA cutoff values were mildly increased.18 Our findings also seem to suggest that prognostication of the optimal timing at when to begin SRT is not independent of factors such as TNM staging and Gleason grading. Gleason scores demonstrated positive predictive significance against 5-year freedom from biochemical recurrence (p = 0.0187). However, postsurgical margin status offered little prognostic value across differing Gleason grades in our cohort. TNM staging did not show statistical significance (p = 0.1932), although our data displayed decreased rates of 5-year biochemical control with worsening stage, as expected.
The foremost goal of our analysis was to determine whether a specific PSA threshold existed at which initiating SRT before said threshold demonstrated superior outcomes in our cohort. A salient improvement in 5-year biochemical control was observed in patients treated with SRT before serum PSA surpassed 0.5 ng/mL compared with patients treated at a pre-radiation therapy PSA value > 0.5 ng/mL. One analysis of 10 retrospective studies and a second multi-institutional retrospective analysis also found that patients treated with SRT at pre-radiation therapy PSA values < 0.5 ng/mL had improved rates of freedom from biochemical failure and that decreasingly lower pre-radiation therapy PSA values among this subset of patients correlated with increasingly improved outcomes.19,20 Other reports have established that SRT employed at PSA ≤ 0.2 ng/mL significantly improves rates of long-term biochemical control and overall patient survival.21,22 A comparison of men with pre-radiation therapy PSA values of ≤ 0.5 ng/mL vs. those with values > 0.5 ng/mL showed the most dramatic difference in 5-year freedom from biochemical failure at 76% vs. 51% (p = 0.0211). Our data supports the rapid initiation of salvage therapy upon identification of biochemical failure. Such a dramatic improvement in biochemical control at the threshold of 0.5 ng/mL suggests that it could be an important target for those encountering such patients in clinical practice.
Conclusions
Salvage postprostatectomy radiation therapy represents a curative treatment option for patients with biochemical recurrence and no evidence of metastases following prostatectomy. The therapeutic advantages of adjuvant radiation therapy and androgen deprivation therapy in the setting of biochemical recurrence are relatively undefined, with prospective studies of this quandary well on the horizon. Adjuvant radiation therapy should continue to be offered to patients exhibiting adverse pathological features for now. Regarding histopathological prognostication, Gleason grading seems to offer the most precision when ascertaining the likelihood of future biochemical recurrence. Initiation of radiation as soon as biochemical failure is identified appears to offer greater success with salvage, particularly when radiation is initiated with PSA ≤ 0.5 ng/mL. Biochemical control appears very durable past the 5-year point, with few late recurrences.
References
- Penson DF, Chan JM, Urologic Diseases in America Project. Prostate cancer. J Urol. 2007;177:2020-2029.
- Pound CR, Partin AW, Eisenberger MA, et. al. Natural history of progression after PSA elevation following radical prostatectomy.JAMA.1999;281(17):1591-1597.
- Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163(6):1632-1642.
- Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. JNCI J Natl Cancer Inst. 2006;98(10):715-717.
- Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14:19-30.
- Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiation therapy after prostatectomy: AUA/ASTRO Guideline.J Urol. 2013;190(2):441-449.
- Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11(1):14-23.
- D’Amico AV, Chen M-H, Sun L, et al. Adjuvant vs. salvage radiation therapy for prostate cancer and the risk of death. BJU Int. 2010;106(11):1618-1622.
- Briganti A, Wiegel T, Joniau S, et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol Internet. 2012;62 (3):472-487.
- Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiation therapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term follow-up of a randomized clinical trial. J Urol. 2009;181(3):956-962.
- Chen C, Lin T, Zhou Y, et al. Adjuvant and salvage radiation therapy after prostatectomy: a systematic review and meta-analysis. PLoS One. 2014;9(8):e104918.
- Pearse M, Fraser-Browne C, Davis ID, et. al. A phase III trial to investigate the timing of radiation therapy for prostate cancer with high-risk features: background and rationale of the Radiation therapy—Adjuvant Vs. Early Salvage (RAVES) trial.BJU Int.2014;113(Suppl 2):7-12.
- Trock BJ. Prostate cancer-specific survival following salvage radiation therapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299(23):2760-2769.
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417-428.
- Siddiqui SA, Boorjian SA, Inman B, et al. Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: a matched cohort study. J Urol. 2008;179:1830.
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiation therapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17(6):747-756.
- Parker C, Clarke N, Logue J, et al. RADICALS (Radiation therapy and androgen deprivation in combination after local surgery). Clin Oncol. 2007;19(3):167-171.
- Briganti A, Karnes RJ, Joniau S, et al. Prediction of outcome following early salvage radiation therapy among patients with biochemical recurrence after radical prostatectomy. Eur Urol. 2014;66(3):479-486.
- Pfister D, Bolla M, Briganti A, et al. Early salvage radiation therapy following radical prostatectomy. Eur Urol. 2014;65 (6):1034-1043.
- Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035-2041.
- Jereczek-Fossa BA, Zerini D, Vavassori A, et al. Sooner or later? Outcome analysis of 431 prostate cancer patients treated with postoperative or salvage radiation therapy. Int J Radiat Oncol. 2009;74(1):115-125.
- Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiation therapy after radical prostatectomy. J Clin Oncol. 2016;34 (30):3648-3654.\
Citation
M S, S P, A B, A P, A V, JB A. Postprostatectomy radiation therapy for biochemically recurrent prostate cancer. Appl Radiat Oncol. 2018;(3):34-39.
September 22, 2018