National Trends in External-Beam Radiation Therapy for Brain Metastases from Lung, Breast, and Melanoma Cancers
Affiliations
- College of Medicine, Drexel University, Philadelphia, PA
- Department of Neurosurgery, Allegheny Health Network, Pittsburgh, PA
- Division of Radiation Oncology, Allegheny Health Network Cancer Institute, Pittsburgh, PA
Abstract
Objective:
Radiation therapy (RT) in the form of stereotactic radiosurgery (SRS) or whole-brain radiation therapy (WBRT) is fundamental for managing brain metastasis (BM). We analyzed national trends in RT and BM patient survival between 2010 and 2019.
Materials and Methods:
The US National Cancer Database was queried for patients receiving RT for BMs who were originally diagnosed with primary non-small cell lung cancer (NSCLC), small cell lung cancer, breast cancer, and melanomas from 2010 to 2019. Patients were grouped by WBRT (5-15 fractions; 20-45 Gy) or SRS (1-5 fractions; 10-40 Gy) treatment. Univariate and multivariate logistic regression analyses identified factors associated with receiving SRS over WBRT. Differences in treatment trends were assessed with Kruskal-Wallis tests. Post-treatment survival was assessed using Kaplan-Meier analysis and a Cox proportional hazards model.
Results:
In total, 59,839 patients were included; 41,197 (68.8%) received WBRT and 18,642 (31.2%) received SRS. Patients who were more recently diagnosed, treated at facilities outside of the East Central regions, insured, diagnosed with NSCLC subtype or melanoma, and who received chemo-/immunotherapy had higher odds of being treated with SRS (all P < .005). SRS, a more recent primary diagnosis, conjunctive use of chemo/immunotherapy, and luminal A/B breast cancer histologies (all P < .01) correlated with increased survival.
Conclusion:
The use of SRS has increased with patient survival over the last decade. We hypothesize that in addition to SRS-reducing neurotoxicity, this increase is due to guideline relaxation, improved techniques, and increased accessibility. Increased patient survival also indicates a possible relationship between SRS usage and improved survival.
Introduction
Brain metastases (BMs) are a common occurrence in approximately 20% to 40% of patients diagnosed with extracranial cancer, which most often include lung cancer, breast cancer, and melanoma.1 BMs are a significant source of mortality and morbidity, with multiple studies reporting mean overall survival less than 1 year following diagnosis.2 - 4 Radiation therapy (RT), either in the form of whole-brain radiation therapy (WBRT) or stereotactic radiosurgery (SRS), has been used to manage BMs either as an individual treatment or in combination with strategies such as surgery, chemotherapy, or immunotherapy, when warranted.
Within the last decade (2010-2019), SRS has become increasingly employed over WBRT when clinically practical, with the aim of minimizing unwanted neurocognitive toxicity associated with WBRT.5 While this general trend is well known, there are limited studies quantifying specific factors associated with SRS usage and the survival of patients treated for BMs within the last decade (2010-2019). Using a national clinical oncology database in the US, we analyzed patterns of WBRT and SRS use and the survival of patients treated for BMs originating from lung, breast, and melanoma primary disease types, which are the leading causes of BMs in the US.3
Materials and Methods
Study Population
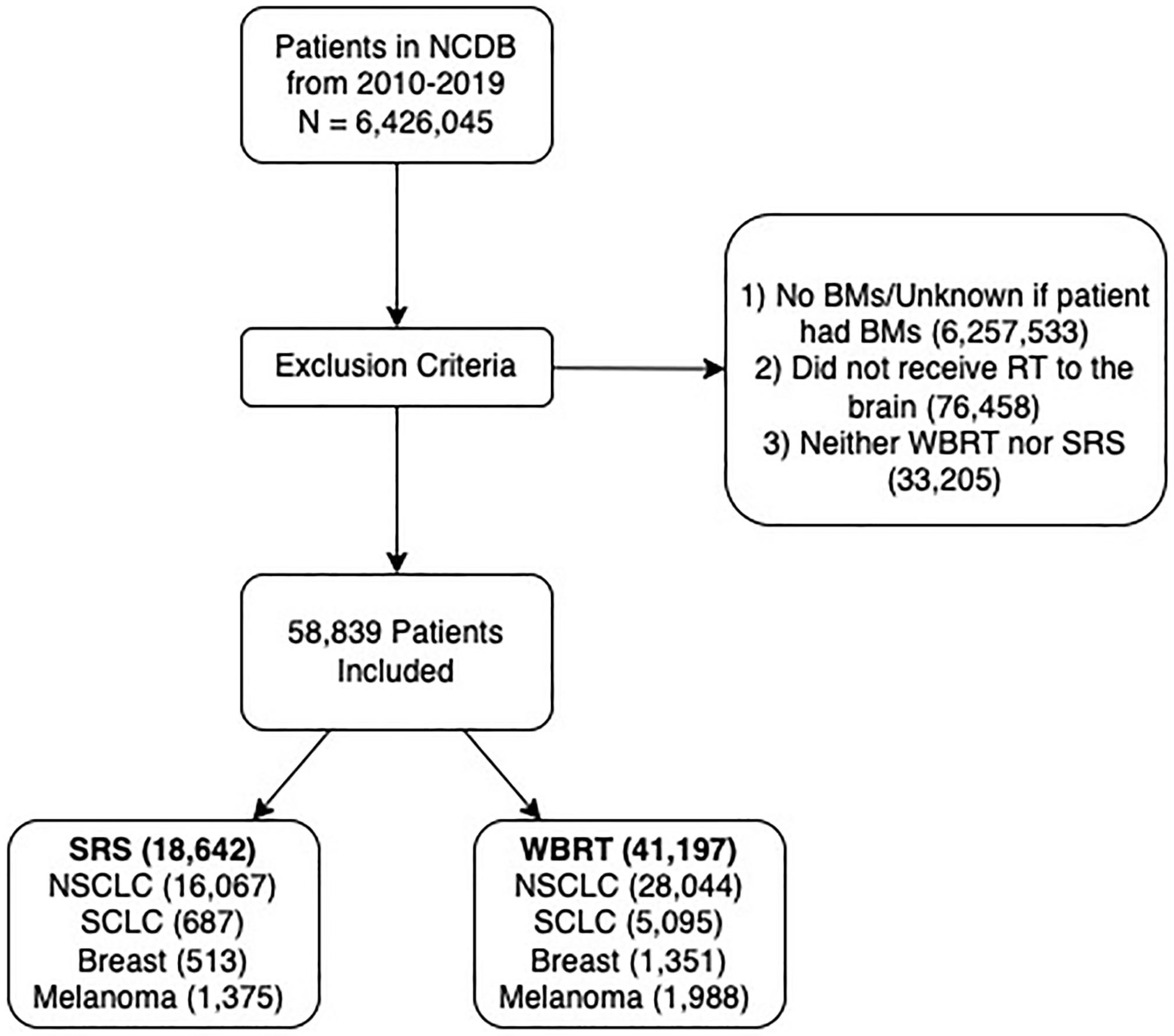
The US National Cancer Database (NCDB) was algorithmically queried to select patients diagnosed with BMs originating from non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), breast, or melanoma-type cancers between 2010 and 2019 ( Figure 1 ). Patients were grouped together based on originating cancer types. The NSCLC patient group was further divided into patients with adenocarcinoma (AC), squamous cell carcinoma (SCC), large cell neuroendocrine carcinoma (LCNEC), or not otherwise specified (NOS) subtypes. The breast cancer patient group was further divided into luminal A, luminal B, triple negative (TN), or HER2-enriched subtypes. For each subtype, patients were then separated by which RT they received (WBRT or SRS). WBRT was defined as 5-15 fractions of RT to the brain, with a total dose of 20-45 Gy. SRS was defined as 1-5 fractions of radiosurgery to the brain to account for techniques similar to SRS such as hypofractionated SRS (fSRS), with a total dose of 10-40 Gy. Patients were excluded from the study if they did not receive brain RT.
Cohort selection flow diagram. The US National Cancer Database (NCDB) was queried to select patients with a brain metastases (BM) diagnosis attributed to non-small cell lung carcinoma (NSCLC), small cell lung carcinoma (SCLC), breast, and melanoma-type cancers from 2010 to 2019. Patients were excluded from the study if they had no radiation therapy (RT) targeting the brain, or whole-brain radiation therapy (WBRT) or stereotactic radiosurgery (SRS) outside our study parameters. Included WBRT patients had total doses of 20-45 Gy and SRS patients had total doses of 10-40 Gy.
Statistical Analysis
Univariate logistic regression was performed on pre-selected patient, disease, geographic, and socioeconomic variables to determine their association with the clinical use of SRS or WBRT. Variables with P < 0.10 in univariate logistic regression analysis were included in a multivariate logistic regression analysis where significance was indicated by P < 0.05. General RT trends were analyzed observationally and graphically. The medians of treatment characteristics between WBRT and SRS were compared using Kruskal-Wallis tests. The same analyses were conducted for each type of RT over each diagnostic year. Kaplan-Meier analysis was performed to determine the probability of survival for each year of diagnosis, RT modality, and primary disease histology, as well as the probability of overall survival for the cohort. A Cox proportional hazards (CPH) model was used to identify survival predictors. Factors that were significant in the univariate CPH analysis ( P < .10) were used in the multivariate CPH model. Significance in the multivariate CPH model was indicated by P < 0.05.
All data organization and analyses were performed using the SPSS Statistics program (version 28.0; IBM) and the following Python (version 3.9.6, Python Software Foundation ) packages: Pandas (version 1.4.1), Scikit-learn (version 0.21), SciPy (version 1.6.0), and Lifelines (0.26.4).
Results
A total of 59,839 patients were included in the study, and of these patients, 41,197 (68.8%) were treated with WBRT and 18,642 (31.2%) were treated with SRS ( Figure 1 ). Of note, patients recently diagnosed, treated at facilities outside of the Midwest, insured, diagnosed with SCC or melanoma, and who received chemo-/immunotherapy were more likely to receive SRS ( Table 1 ). In contrast, patients diagnosed with SCLC, LCNEC, and breast cancers, as well as patients presenting with extracranial metastases, were more likely to receive WBRT.
Variables Significant in Univariate Logistic Regression Analysis of Stereotactic Radiosurgery (SRS) Usage ( P < .10), Presented With Multivariate Logistic Regression Results ( P < .05)
| VARIABLES | LOGISTIC REGRESSION MODEL | |||||||
|---|---|---|---|---|---|---|---|---|
| N | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | ||||||
| WBRT | SRS | OR | P VALUE | 95% CI | OR | P VALUE | 95% CI | |
| Age | - | - | 1.01 | < .005 | 1.01-1.01 | 1.02 | < .005 | 1.01-1.02 |
| Race | ||||||||
| White | 34,722 | 15,662 | Reference | Reference | ||||
| Black | 4783 | 1999 | .93 | .007 | 0.88-0.98 | .96 | .21 | .90-1.02 |
| Other | 1692 | 981 | 1.29 | < .005 | 1.19-1.39 | 1.04 | .374 | .95-1.15 |
| Year of diagnosis | ||||||||
| 2010 | 3921 | 623 | Reference | Reference | ||||
| 2011 | 3974 | 742 | 1.18 | .006 | 1.05-1.32 | 1.22 | < .005 | 1.08-1.38 |
| 2012 | 4121 | 905 | 1.38 | < .005 | 1.24-1.54 | 1.42 | < .005 | 1.26-1.60 |
| 2013 | 4137 | 1123 | 1.71 | < .005 | 1.53-1.90 | 1.71 | < .005 | 1.52-1.92 |
| 2014 | 4071 | 1297 | 2.01 | < .005 | 1.81-2.23 | 1.92 | < .005 | 1.71-2.15 |
| 2015 | 3660 | 1517 | 2.61 | < .005 | 2.35-2.89 | 2.58 | < .005 | 2.30-2.89 |
| 2016 | 4537 | 2381 | 3.30 | < .005 | 2.99-3.64 | 3.40 | < .005 | 3.06-3.79 |
| 2017 | 4207 | 2824 | 4.22 | < .005 | 3.83-4.66 | 4.29 | < .005 | 3.85-4.78 |
| 2018 | 4451 | 3552 | 5.02 | < .005 | 4.57-5.52 | 5.11 | < .005 | 4.58-5.69 |
| 2019 | 4118 | 3678 | 5.62 | < .005 | 5.11-6.18 | 5.81 | < .005 | 5.20-6.48 |
| Community type | ||||||||
| Metro | 33,568 | 15,759 | Reference | Reference | ||||
| Urban | 6646 | 2514 | .81 | < .005 | .77-.85 | .87 | < .005 | .82-.93 |
| Rural | 983 | 369 | .80 | < .005 | .71-.91 | .92 | .289 | .80-1.07 |
| Location | ||||||||
| East North Central | 9021 | 3236 | Reference | Reference | ||||
| East South Central | 3732 | 1239 | .93 | .045 | .85-.99 | 1.09 | .078 | .99-1.19 |
| Mid-Atlantic | 5745 | 3398 | 1.65 | < .005 | 1.56-1.75 | 1.73 | < .005 | 1.61-1.85 |
| Mountain | 1319 | 747 | 1.58 | < .005 | 1.43-1.74 | 1.68 | < .005 | 1.50-1.89 |
| New England | 2855 | 1313 | 1.28 | < .005 | 1.19-1.38 | 1.38 | < .005 | 1.26-1.51 |
| Pacific | 3466 | 1776 | 1.40 | < .005 | 1.31-1.51 | 1.44 | < .005 | 1.32-1.56 |
| South Atlantic | 7982 | 3840 | 1.34 | < .005 | 1.27-1.42 | 1.42 | < .005 | 1.33-1.52 |
| West North Central | 4068 | 1713 | 1.17 | < .005 | 1.09-1.25 | 1.19 | < .005 | 1.10-1.30 |
| West South Central | 2587 | 1071 | 1.15 | < .005 | 1.07-1.27 | 1.22 | < .005 | 1.11-1.35 |
| Unknown | 422 | 309 | 2.04 | < .005 | 1.75-2.38 | 2.62 | < .005 | 2.16-3.17 |
| Insurance status | ||||||||
| Uninsured | 2111 | 469 | Reference | Reference | ||||
| Private insurance | 13,417 | 6485 | 2.18 | < .005 | 1.96-2.41 | 1.95 | < .005 | 1.72-2.20 |
| Government insurance | 25,647 | 11,677 | 2.05 | < .005 | 1.85-2.27 | 1.67 | < .005 | 1.48-1.88 |
| Unknown | 22 | 11 | 2.25 | .029 | 1.08-4.67 | 1.05 | .910 | .48-2.27 |
| Histology | ||||||||
| NSCLC—adeno | 20,219 | 11,818 | Reference | Reference | ||||
| NSCLC—SCC | 2906 | 2098 | 1.24 | < .005 | 1.16-1.31 | 1.43 | < .005 | 1.33-1.53 |
| NSCLC—LCNEC | 928 | 220 | .41 | < .005 | 0.35-0.47 | .38 | < .005 | .32-.45 |
| NSCLC—NOS | 3991 | 1929 | .83 | < .005 | .78-.88 | 1.06 | < .005 | .99-1.14 |
| SCLC | 10,123 | 687 | .12 | < .005 | .11-.13 | .10 | < .005 | .09-.11 |
| Breast—luminal A | 428 | 110 | .44 | < .005 | .36-.54 | .61 | < .005 | .48-.78 |
| Breast—luminal B | 195 | 48 | .42 | < .005 | .31-.58 | 0.43 | < .005 | .20-0.61 |
| Breast—HER2 | 141 | 51 | .62 | < .005 | .45-.85 | .62 | .012 | .43-.90 |
| Breast—TN | 262 | 72 | .74 | .062 | .53-1.02 | .66 | .005 | .50-.89 |
| Breast—NOS | 461 | 232 | .68 | .096 | .43-1.07 | .57 | < .005 | .48-.68 |
| Melanoma | 1543 | 1375 | 1.76 | < .005 | 1.56-1.98 | 1.75 | < .005 | 1.59-1.92 |
| Distance to hospital (miles) | - | - | 1.00 | < .005 | 1.00-1.00 | 1.00 | < .005 | 1.00-1.00 |
| Chemotherapy | ||||||||
| No | 15,325 | 6649 | Reference | Reference | ||||
| Yes | 25,872 | 11,993 | 1.07 | < .005 | 1.03-1.11 | 1.60 | < .005 | 1.53-1.68 |
| Immunotherapy | ||||||||
| No | 35,459 | 12,691 | Reference | Reference | ||||
| Yes | 5738 | 5951 | 2.90 | < .005 | 2.78-3.02 | 1.47 | < .005 | 1.39-1.56 |
| Extracranial metastases* | ||||||||
| No | 41,048 | 18,540 | Reference | Reference | ||||
| Yes | 149 | 102 | 1.52 | < .005 | 1.18-1.95 | .59 | < .005 | .43-.79 |
Abbreviations: CI, confidence interval; OR, odds ratio; Adeno, adenocarcinoma; NSCLC, non-small cell lung cancer; WBRT, whole-brain radiation therapy; SCC, squamous cell carcinoma; SCLC, small cell lung carcinoma; LCNEC, large cell neuroendocrine carcinoma; TN, triple negative; NOS, not otherwise specified.
*Bone, lung, liver.
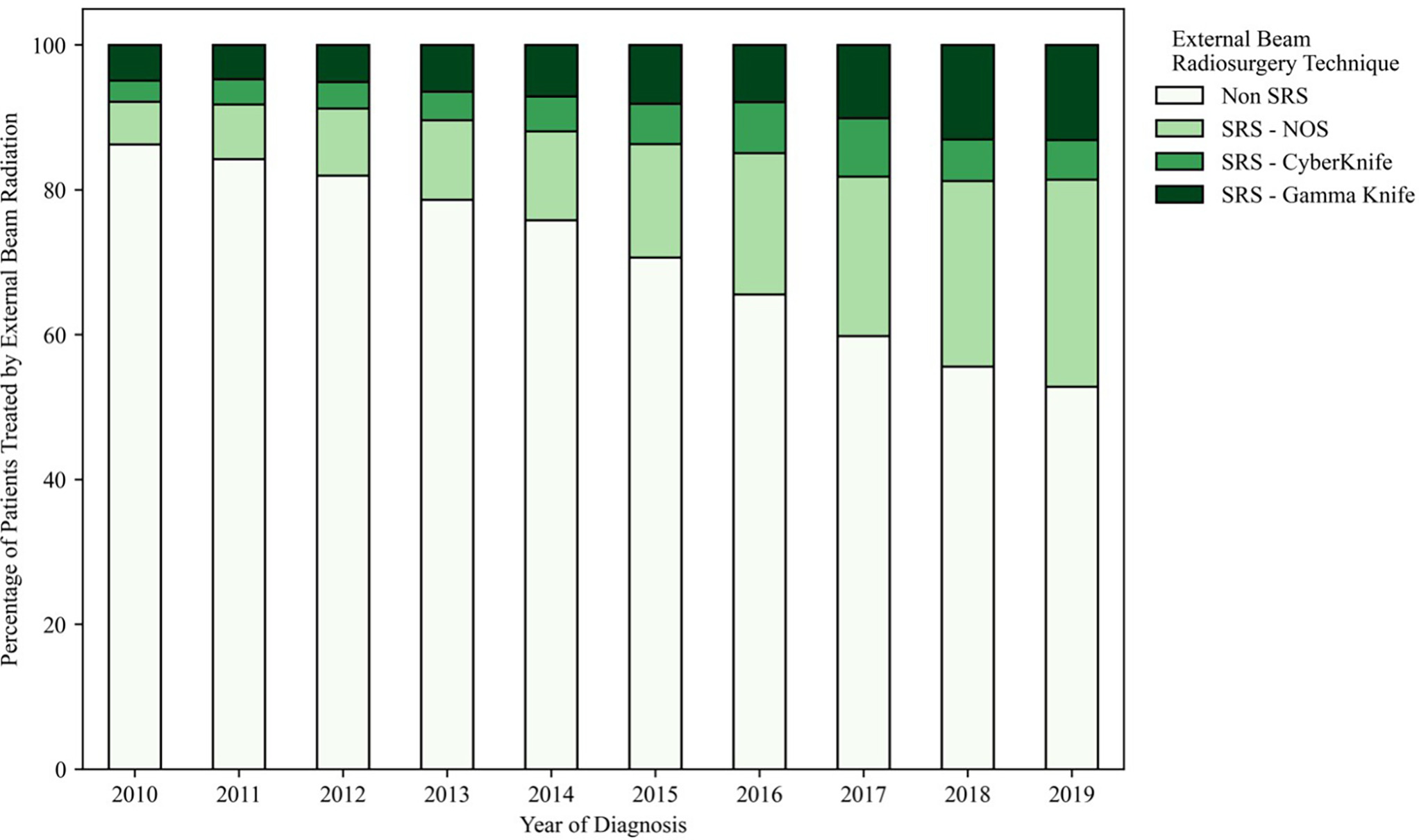
From 2010 to 2019, there was an increase in the time from diagnosis to treatment and a shift in the use of some treatments over others. The use of SRS increased: It was used to treat only 13.7% of cases in 2010 compared with 47.2% in 2019 ( Figure 2 ). Of SRS-specific technologies, Gamma Knife (GK) (Elekta) usage has increased relative to CyberKnife (CK) (Accuray) usage (167 % increase in GK usage vs an 86% increase in CK usage between 2010 and 2019). Non-GK and non-CK SRS modalities relatively increased the most during this study period (385% increase).
Relative usage of stereotactic radiosurgery (SRS) (out of all external-beam radiation therapy [RT]) in the US from 2010 to 2019 for the treatment of non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), breast cancers, and melanomas. NOS, not otherwise specified.
Median time from diagnosis to RT for WBRT was 17 days compared with 34 days for SRS treatments ( P < .005; Table 2 ). From 2010 to 2019, the median time from diagnosis to RT increased from 15 days to 20 days for WBRT ( P < .005; Table 3 ) and from 32 days to 35 days for SRS ( P = .014; Table 4 ). Neither dose nor fractions changed appreciably over this time range for either treatment modality.
Mann-Whitney U Analysis Comparing Median Treatment Characteristics for Whole-Brain Radiation Therapy (WBRT) and Stereotactic Radiosurgery (SRS)
| RADIATION THERAPY TYPE | MEDIAN DAYS FROM DX TO RADIATION ( P < .005) | MEDIAN TOTAL DOSE ( P < .005) | MEDIAN FRACTIONS ( P < .005) |
|---|---|---|---|
| WBRT | 17 (7-34) | 30 Gy (20-45) | 10 (6-15) |
| SRS | 34 (22-53) | 21 Gy (10-40) | 1 (1-5) |
Abbreviation: Dx, diagnosis.
Kruskal-Wallis Analysis Comparing Median Stereotactic Radiosurgery Treatment Characteristics from 2010 to 2019
| YEAR OF DIAGNOSIS | MEDIAN DAYS FROM DX TO RADIATION ( P = .014) | MEDIAN TOTAL DOSE ( P < .005) | MEDIAN FRACTIONS ( P < .005) |
|---|---|---|---|
| 2010 | 32 (20-51) | 20 Gy (11-40) | 1 (1-5) |
| 2011 | 34 (21-54) | 20 Gy (11-40) | 1 (1-5) |
| 2012 | 33 (21-49) | 20 Gy (11.7-40) | 1 (1-5) |
| 2013 | 33 (22-52) | 20 Gy (10-40) | 1 (1-5) |
| 2014 | 35 (22-53) | 20 Gy (10-40) | 1 (1-5) |
| 2015 | 35 (22-52) | 20 Gy (10-40) | 1 (1-5) |
| 2016 | 34 (22-53) | 21 Gy (10-40) | 1 (1-5) |
| 2017 | 34 (22-52) | 21 Gy (10-40) | 1 (1-5) |
| 2018 | 35 (22-53) | 21 Gy (10-40) | 1 (1-5) |
| 2019 | 35 (22-54) | 21 Gy (10-40) | 1 (1-5) |
Abbreviation: Dx, diagnosis.
Kruskal-Wallis Analysis Comparing Median Whole-Brain Radiation Therapy Treatment Characteristics from 2010 to 2019
| YEAR OF DIAGNOSIS | MEDIAN DAYS FROM DX TO RADIATION ( P < .005) | MEDIAN TOTAL DOSE ( P < .005) | MEDIAN FRACTIONS ( P < .005) |
|---|---|---|---|
| 2010 | 15 (6-31) | 30 (20-45) | 10 (6-15) |
| 2011 | 15 (6-32) | 30 (20-45) | 10 (6-15) |
| 2012 | 15 (6-30) | 30 (20-45) | 10 (6-15) |
| 2013 | 17 (7-33) | 30 (20-45) | 10 (6-15) |
| 2014 | 16 (7-31) | 30 (20-45) | 10 (6-15) |
| 2015 | 17 (7-33) | 30 (20-45) | 10 (6-15) |
| 2016 | 18 (7-34) | 30 (20-45) | 10 (6-15) |
| 2017 | 19 (8-35) | 30 (20-45) | 10 (6-15) |
| 2018 | 19 (8-36) | 30 (20-45) | 10 (6-15) |
| 2019 | 20 (8-39) | 30 (20-45) | 10 (6-15) |
Abbreviation: Dx, diagnosis.
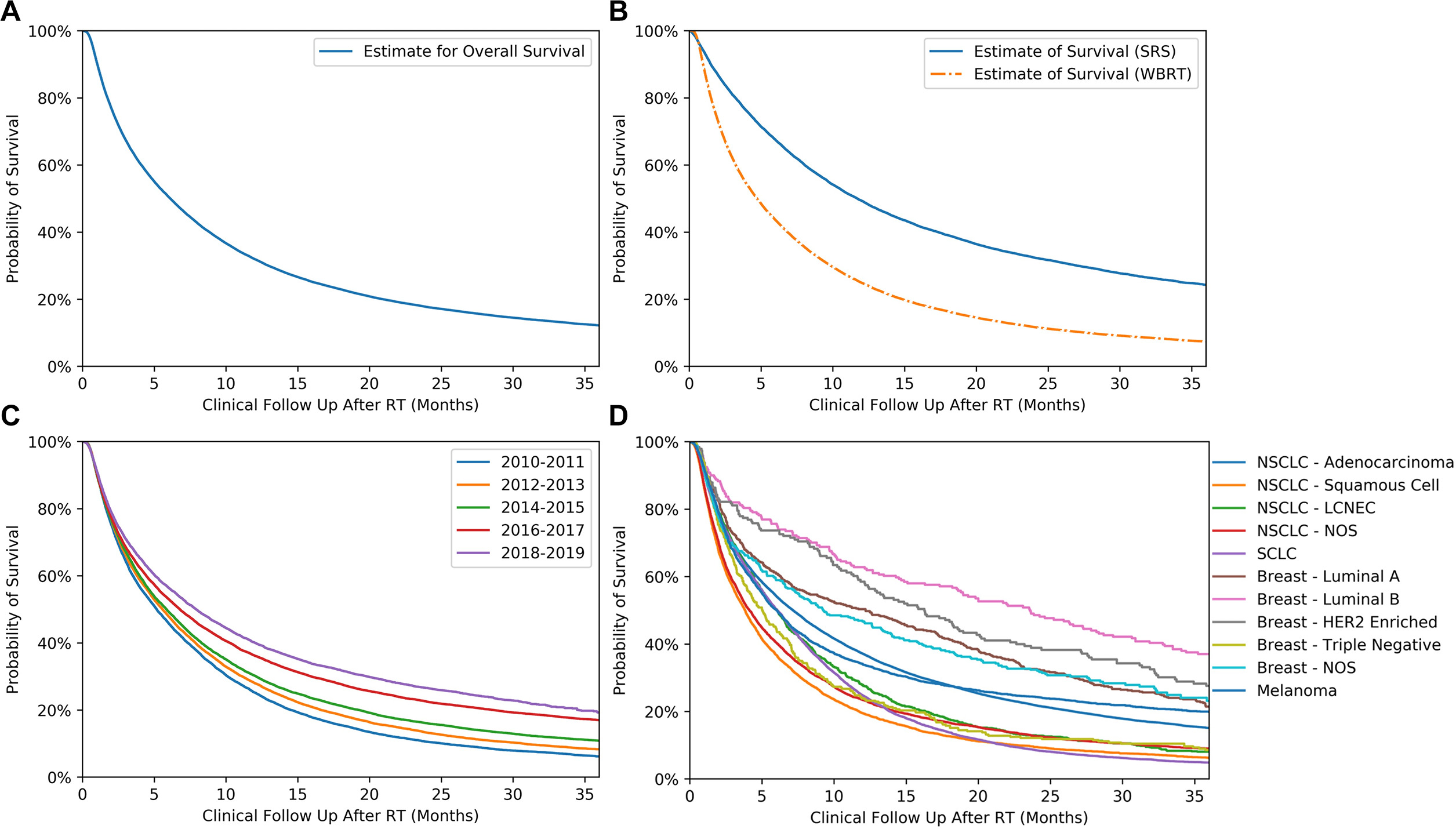
We also compared survival times for the different cohorts. Median overall survival for all patients ( Figure 3A ) was 6.11 months (CI: 6.01-6.21). Patients receiving WBRT had a median survival of 4.73 months (CI: 4.57-4.89), and patients receiving SRS had a median survival of 11.72 months (CI: 11.50-11.91) ( P < .005; Figure 3B ). Median survival consistently increased over the decade from 5.19 months (CI: 5.00-5.38) in 2010-2011 to 7.92 months (CI: 7.59-8.27) in 2018-2019 ( P < .005; Figure 3C ). Of the disease histologies, SCC had the lowest median survival at 3.84 months (CI: 3.65-4.00), and luminal B breast cancer had the highest median survival at 23.56 months (CI: 18.59-28.35). All disease survival rates can be seen in Figure 3 ( P < .005).
Kaplan-Meier curves depicting estimates of survival following radiation therapy (RT) treatment by overall cohort ( A ), RT modality ( B ), year of primary diagnosis ( C ), and primary histology ( D ). NSCLC, non-small cell lung cancer; LCNEC, large cell neuroendocrine carcinoma; NOS, not otherwise specified.
Multivariate CPH analysis revealed that increased survival correlated with SRS treatment (hazard ratio [HR]: 0.50 CI [0.49-0.52]), recent years of primary diagnosis (2018-2019 hour: 0.67 CI [0.65-0.69]), chemotherapy (HR: 0.49 CI [0.48-0.50]), and immunotherapy (HR: 0.58 CI [0.55-0.60]) in conjunction with RT, luminal A (HR: 0.67 CI [0.62-0.75]), and luminal B (HR: 0.76 CI [0.66-0.91]) breast histologies. In contrast, decreased survival correlated with WBRT treatment (HR: 2.00 CI [1.92-2.04]), unknown insurance status (HR: 1.25 CI [1.13-2.79]), and certain histologies NSCLC-SCC (HR: 1.40 CI [1.33-1.44]), NSCLC-LCNEC (HR: 1.16 CI [1.07-1.24]), NSCLC-NOS (HR: 1.26 CI [1.22-1.30]), SCLC (HR: 1.31 CI [1.27-1.34]), and breast-TN (HR: 1.43 CI [1.28-1.63]) histologies. Results of the survival analysis can be seen in Table 5 .
Variables Significant in the Cox Proportional Hazards Model (CPH) of Survival ( P < .10), Presented With Multivariate CPH Model Results ( P < .05)
| VARIABLE | COX PROPORTIONAL HAZARDS MODEL | |||||
|---|---|---|---|---|---|---|
| UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | |||||
| HR | P VALUE | 95% CI | OR | P VALUE | 95% CI | |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 0.84 | < .005 | 0.83-0.86 | 0.88 | < .005 | 0.87-0.90 |
| Age | ||||||
| Analyzed continuously | 1.02 | < .005 | 1.02-1.02 | 1.01 | < .005 | 1.01-1.01 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 0.93 | .007 | 0.88-0.98 | 0.92 | < .005 | 0.89-0.96 |
| Other | 1.29 | < .001 | 1.19-1.39 | 0.77 | < .005 | 0.73-0.82 |
| Year of diagnosis | ||||||
| 2010-2011 | Reference | Reference | ||||
| 2012-2013 | 0.92 | < .005 | 0.90-0.95 | 0.95 | < .005 | 0.93-0.98 |
| 2014-2015 | 0.86 | < .005 | 0.83-0.88 | 0.92 | < .005 | 0.89-0.94 |
| 2016-2017 | 0.72 | < .005 | 0.70-0.74 | 0.78 | < .005 | 0.76-0.80 |
| 2018-2019 | 0.65 | < .005 | 0.63-0.67 | 0.67 | .02 | 0.65-0.69 |
| Community type | ||||||
| Metro | Reference | Reference | ||||
| Urban | 1.12 | < .005 | 1.09-1.15 | 1.23 | < .005 | 1.09-1.39 |
| Rural | 1.16 | < .005 | 1.09-1.24 | 1.30 | < .005 | 1.15-1.47 |
| Location | ||||||
| East North Central | Reference | Reference | ||||
| East South Central | 0.88 | < .005 | 0.84-0.91 | 0.91 | < .005 | 0.87-0.96 |
| Mid-Atlantic | 1.04 | 8.00E-02 | 1.00-1.07 | 1.07 | < .005 | 1.02-1.12 |
| Mountain | 1.05 | 1.00E-02 | 1.01-1.09 | 1.09 | < .005 | 1.05-1.15 |
| New England | 1.17 | < .005 | 1.12-1.23 | 1.12 | < .005 | 1.06-1.18 |
| Pacific | 1.07 | < .005 | 1.02-1.12 | 1.11 | < .005 | 1.06-1.17 |
| South Atlantic | 0.97 | 2.20E-01 | 0.92-1.02 | 0.98 | .45 | 0.92-1.04 |
| West North Central | 0.96 | 1.80E-01 | 0.90-1.02 | 0.96 | .30 | 0.90-1.03 |
| West South Central | 0.92 | < .005 | 0.88-0.96 | 0.97 | .22 | 0.92-1.02 |
| Unknown | 0.52 | < .005 | 0.47-0.58 | 1.05 | .44 | 0.93-1.18 |
| Insurance status | ||||||
| Uninsured | Reference | Reference | ||||
| Private insurance | 0.75 | < .005 | 0.71-0.78 | 0.82 | < .005 | 0.77-0.86 |
| Government insurance | 1.04 | .06 | 1.00-1.09 | 0.88 | < .005 | 0.84-0.93 |
| Unknown | 1.25 | .28 | 0.84-1.87 | 1.75 | .01 | 1.13-2.70 |
| Charlson-Deyo score | ||||||
| 0 | Reference | Reference | ||||
| 1 | 1.22 | < .005 | 1.19-1.25 | 1.11 | < .005 | 1.08-1.14 |
| 2+ | 1.35 | < .005 | 1.31-1.39 | 1.17 | < .005 | 1.13-1.21 |
| Histology | ||||||
| NSCLC—adeno | Reference | Reference | ||||
| NSCLC—squamous | 1.58 | < .005 | 1.53-1.63 | 1.40 | < .005 | 1.33-1.44 |
| NSCLC—large cell neuroendocrine | 1.25 | < .005 | 1.17-1.34 | 1.16 | < .005 | 1.07-1.24 |
| NSCLC—NOS | 1.39 | < .005 | 1.35-1.44 | 1.26 | < .005 | 1.22-1.30 |
| SCLC | 1.35 | < .005 | 1.32-1.39 | 1.31 | < .005 | 1.27-1.34 |
| Breast—luminal A | 0.83 | < .005 | 0.76-0.91 | 0.67 | < .005 | 0.62-0.75 |
| Breast—luminal B | 0.57 | < .005 | 0.50-0.66 | 0.76 | < .005 | 0.66-0.91 |
| Breast—HER2 enriched | 0.67 | < .005 | 0.57-0.79 | 1.03 | .74 | 0.88-1.25 |
| Breast—TN | 1.3 | < .005 | 1.16-1.46 | 1.43 | < .005 | 1.28-1.63 |
| Breast—NOS | 0.81 | < .005 | 0.72-0.91 | 0.99 | .90 | 0.85-1.21 |
| Melanoma | 0.94 | .01 | 0.90-0.99 | 0.79 | < .005 | 0.75-0.84 |
| Distance to hospital (miles) | ||||||
| Analyzed continuously | 1.00 | < .005 | 1.00-1.00 | 1.00 | .01 | 1.00-1.00 |
| Chemotherapy | ||||||
| No | Reference | Reference | ||||
| Yes | 0.54 | < .005 | 0.53-0.55 | 0.49 | < .005 | 0.48-0.50 |
| Immunotherapy | ||||||
| No | Reference | Reference | ||||
| Yes | 0.54 | < .005 | 0.52-0.56 | 0.58 | < .005 | 0.55-0.60 |
| Experimental therapy | ||||||
| No | Reference | Reference | ||||
| Yes | 0.84 | < .005 | 0.75-0.95 | 0.88 | .05 | 0.77-1.00 |
| External radiation therapy type | ||||||
| WBRT | Reference | Reference | ||||
| SRS | 0.52 | < .005 | 0.51-0.53 | 0.50 | < .005 | 0.49-0.52 |
Abbreviations: CI, confidence interval; OR, odds ratio; Adeno, adenocarcinoma; WBRT, whole-brain radiation therapy; SRS, stereotactic radiosurgery; SCC, squamous cell carcinoma; LCNEC, large cell neuroendocrine carcinoma; TN, triple negative; NOS, not otherwise specified.
Discussion
We found in this large database analysis that SRS is being increasingly utilized for the management of brain metastases. We found several factors, including updated clinical guidelines, regional availability, and the use of SRS-adjacent techniques, to be associated with increased utilization. One such SRS-adjacent technique is fSRS, which combines the steep-dose gradients and smaller margins of SRS with the radiobiologic advantages of fractionation. The odds of receiving SRS also vary by primary disease type and socioeconomic factors, such as insurance status and proximity to metropolitan environments.
Over the past decade, SRS has been increasingly employed to minimize the risk of neurocognitive decline associated with WBRT while still maintaining similar survival rates for patients with 3 BMs.6 - 8 Within the last couple of years, however, its use has been expanded to patients with 4-15 BMs, where WBRT may traditionally have been used.9 Several studies, including a large-scale retrospective review in 2020 and a meta-analysis in 2022, support the effectiveness of first-line SRS in patients with a primary SCLC diagnosis who would have otherwise been treated with WBRT.10 , 11 Continued evidence depicting SRS’s decreased parenchymal tissue damage and comparable effectiveness to WBRT supports relaxing the clinical guidelines and promoting the widespread adoption of SRS over time. Additionally, the rise of frameless GK treatment reduces the logistical constraints and increases patient satisfaction, further explaining the observed increase in SRS use.12 , 13 Furthermore, adopting fSRS allows for the treatment of metastases that are large or in unfavorable locations.14
Our study also demonstrated that regional and socioeconomic discrepancies determine a patient’s odds of receiving SRS. A 2012 Canadian study found that on-site SRS availability was the most important factor in receiving SRS treatment.15 The distribution of SRS systems was surveyed in 2019, and researchers found that most states in the United States have a ratio of at least 1 SRS machine (GK,linear accelerator, CK) per 1,000,000 people, with a few exceptions. Several states in the South Atlantic, West North Central, West South Central, and New England regions had ratios less than 1, while Vermont, South Dakota, and Wyoming all had 0 machines per 1,000,000 people.16 Even within certain geographic regions of the United States, proximity to a metropolitan area correlates with higher odds of being treated with SRS in addition to a higher probability of survival.17 Likewise, multiple studies concur that insured patients have much higher odds of receiving SRS and a higher probability of survival compared with uninsured populations.18 - 20 However, lower survival in uninsured patients and those distant from metro centers may be confounded by an increased disease burden at clinical presentation from a lack of preventative and continued health care.
Scenarios in which patients receive WBRT include extensive intracranial disease, multiple distant failures, or poor performance status.7 As a result of these confounding variables, we observed that the risk of mortality was almost halved at the post-treatment 3-year mark among patients who were treated with SRS compared with WBRT. However, a recent 2021 study showed that short-term survival rates are significantly higher for SRS patients than WBRT patients (1-year survival; SRS = 46.4% vs WBRT = 38.8%), when examining crude mortality between a propensity-matched SRS and WBRT cohort.21 Another study in 2016 claimed that SRS had a larger survival benefit than WBRT in the first 6 months after treatment for postoperative resection cavities ( P = .003).22 In our study, we also observed patients living longer with their diagnoses as median overall survival increased by 50% over the last decade. With patients’ post-diagnosis life expectancy increasing, the long-term neurocognitive effects of WBRT must be given greater consideration when developing treatment plans. Investigating the long-term survival rates and neurocognitive preservation for different RT strategies is therefore warranted in future studies.
Primary tumor subtype remains an important prognostic factor for BM patients. In consensus with existing literature, our study found that breast cancer patients (excluding TN subtypes) survived the longest following treatment.23 , 24 Within breast cancer subtypes, patients with luminal B and HER2 diagnoses demonstrated the greatest survival, both in this analysis and the literature.25 Improved survival in BMs associated with HER2-positive breast cancer may be associated with the advent of newer agents such as Enhertu (T-DXd). T-DXd has been proven to have intracranial activity with minimal toxicity when treating advanced breast cancer patients with BMs.26 Interestingly, within lung cancer subtypes, patients with SCLC had better survival following treatment than patients with SCC or NOS NSCLC subtypes. Other studies support that patients with SCLC have an increased risk of developing BMs; however, there is no evidence suggesting survival rates are lower among this subgroup compared with other lung cancer subtypes.27
This study has several limitations to consider when interpreting results. The NCDB has limited available data, so all analyses used phase I RT data, which do not account for subsequent treatments, such as salvage SRS or WBRT. Furthermore, the NCDB lacks data on the size, number, or location of BMs as well as prognostic factors, such as performance score or tumor grade. It also does not include BM-specific surgical data, even though surgery remains an important treatment for BMs. Additionally, there is a lack of information regarding other downstream effects of BM treatments in the literature, such as leptomeningeal disease and radiation necrosis. Finally, because patients were partitioned into the general SRS and WBRT groups, it is possible that some treated with unconventional or experimental doses/fractions were excluded.
SRS usage over the last decade has increased nationwide due to relaxation of guidelines, improved techniques, and accessibility of technology. The increase in patient survival over this same period indicates a possible relationship between SRS usage and improved survival. Finally, patient characteristic discrepancies in RT usage should be explored to identify ways to overcome BM treatment limitations.
References
Citation
Rajkumar S, Desai J, Shepard MJ, Wegner RE. National Trends in External-Beam Radiation Therapy for Brain Metastases from Lung, Breast, and Melanoma Cancers. Appl Radiat Oncol. 2024;(1):39 - 49.
doi:10.37549/ARO-D-23-00030
March 1, 2024