Magnetic Appeal: Changing Treatment Paradigms with MR-Guided Radiation Therapy
Images
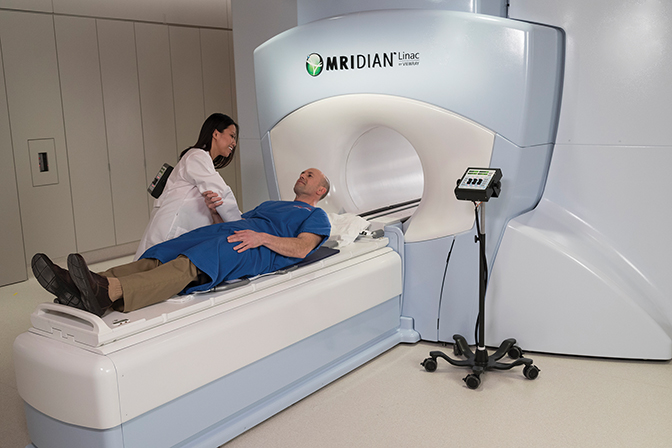
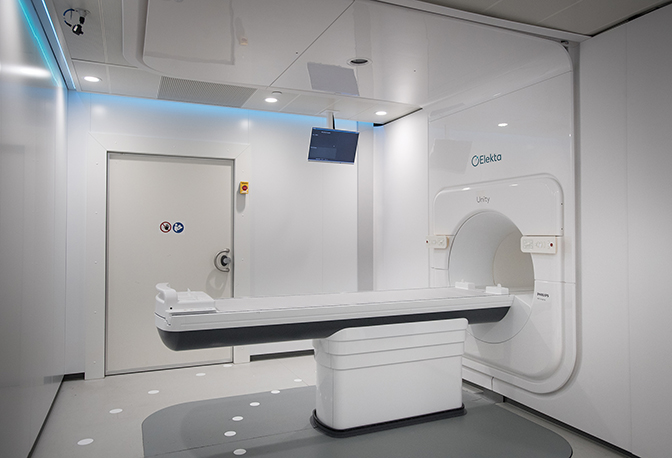
With an armamentarium of nearly every state-of-the-art radiation therapy technology under one roof, Baptist Health’s Miami Cancer Institute was one of the first sites to implement an MR-guided linac in April 2018. Michael Chuong, MD, medical director of the Proton Therapy Center, physician director of the MRI-Guided Radiation Therapy Program, and director of Radiation Oncology Clinical Research, was one of the first physicians to treat patients using the new technology.
“The ability to visualize the internal anatomy of patients, not just prior to, but also continuously during treatment, and perform on-table adaptive replanning as needed, is really unique among our other advanced radiation therapy technologies,” says Dr. Chuong. “We’ve tried to push the envelope to benefit patients and that is through significant dose escalation for a large percentage of our patients.”
MR-guided radiation therapy (MRgRT), offered through two primary companies – ViewRay (MRIdian) (Figure 1) and Elekta (Elekta Unity) (Figure 2) – allows users to adjust radiation dose in real-time based on live MR images of the tumor and surrounding anatomy. This MR guidance is “a fundamental paradigm shift that will be more broadly adopted in the future,” says Dr. Chuong, “especially as costs and treatment times decrease.”
Early Adopters
At Miami Cancer Institute, which uses MRIdian, pancreatic cancer is the primary disease site treated, specifically to escalate dose to an ablative range; the most common dose fractionation schedule is 50 Gy/5 fractions. At the 2021 annual congress of the European Society for Radiotherapy and Oncology (ESTRO), Dr. Chuong presented results of an analysis of 50 patients treated at the institute suggesting that ablative MRgRT could improve long-term local tumor control and overall survival. While the median overall survival after chemotherapy and conventional radiation therapy is about 12 to 15 months, patients in the study achieved median overall survival of 21 months, with a 50% survival rate after 2 years.1
Sunnybrook’s Odette Cancer Centre was one of the first users of Elekta Unity, initially installing a prototype, says Arjun Sahgal, MD, director of the MR-linac program and deputy chief of radiation oncology. In August 2019, Sunnybrook enrolled its first patient in the MOMENTUM (The Multiple OutcoMe EvaluatioN of radiation Therapy Using the MR-linac) study, which aims to accelerate the technical and clinical development of anatomic and functional MRgRT and facilitate the evidence-based introduction of the MR-linac into clinical practice.2 Sunnybrook is a founding member of the Elekta MR-Linac Consortium, a collaborative industrial-academic partnership developed to support advancement of the technology) and 1 of 8 international centers involved in MOMENTUM. Although the center is focused on central nervous system tumors, they have treated prostate cancer and will soon treat pancreatic cancer and head and neck patients.
Time and Workload Needs
A disadvantage of current MRgRT therapies is the added time required for treatment. That’s primarily because while the patient is on the MR-linac table, the images are acquired, then the tumor is contoured from those daily images, and the treatment plan is adapted as needed. Following treatment plan generation and physics quality checks, the patient is treated. Auto-contouring, faster systems for computation, and migrating to volumetric-modulated radiation therapy (VMAT) should help reduce that time, Dr. Sahgal says.
However, with MRgRT, part of the weakness is also a strength. “The key is you want to be able to treat the tumor of the day,” he says. “Even in the brain, we have observed and reported on tumor migration.3 Understanding that migration during treatment is key to building that next phase of radiation oncology, which is clinical target volume (CTV) margin reduction. The only way we are going to achieve that is by imaging each day prior to radiation delivery with MR. Understanding where the microscopic disease is and how we can shrink the margin safely will lead to less normal tissue being irradiated.”
After treating more than 150 brain tumors with the MR-linac, this is precisely what Sunnybrook is doing with the UNITED study: evaluating the safety of reducing CTV margins for glioblastoma patients from 1.5 cm to 5 mm with weekly adaption of the treatment plan.4 If successful, the next step is incorporating metabolic imaging and voxel-based dose escalation to areas of resistant tumor.
Physician workload also expands with use of the technology. “We no longer work in the background, contouring and reviewing plans for approval, and seeing the patient weekly in review clinics,” Dr. Sahgal adds. “We are there in the treatment room, doing the procedure even multiple times during a patient’s course of therapy and essentially creating personalized treatments much akin to what a surgeon does. Our role is becoming more complex and in the moment.”
Two Systems, Different Approaches
The development of Elekta Unity followed a concept originally proposed by Jan Lagendijk, PhD, and Bas W. Raaymakers, PhD, at University Medical Center (UMC) Utrecht (Netherlands): integrating a linac and MRI by creating a “donut”-type gap within the magnetic field, thus creating a magnetic-field-free zone where the linac is then placed.5 Elekta collaborated with UMC Utrecht and Philips Healthcare to commercialize that concept, says John Christodouleas, MD, senior vice president of medical affairs and clinical research at Elekta, and a radiation oncologist at the Hospital of the University of Pennsylvania.
The inclusion of a 1.5T MR “opens up the field to a larger breadth of technologies already developed in the diagnostic realm, where 70% of all MRIs are 1.5T,” Dr. Christodouleas says. He notes that online-guided radiation therapy represents less than 1% of the global radiation therapy market. Elekta has 42 systems installed or about to go live, 11 of which are in the US.
A benefit of using a higher-field-strength MR system is a greater signal-to-noise ratio, which can increase image quality, reduce background noise and shorten scanning time. “These devices are meant to support adaptive paradigms,” he adds. “[MRgRT] is being used anywhere the clinician needs ultraprecision or the capability to adapt to changes in anatomy or biology.”
The Elekta Unity delivers 3 distinct adaptive paradigms, explains Dr. Christodouleas. Most people will think about adapting to the anatomy, including the shape and position of the tumor in relationship to surrounding healthy tissue. However, the ability to image the patient in real time during treatment also enables the clinician to see and adapt the actual dosimetry delivered to the patient. Additionally, the clinician can see the biology and adapt treatment to the patient’s response.
“We’ve been adjusting for changes in shape, but dose adaptive and response adaptive are concepts that have been hard to act upon,” he says. These are areas of enormous interest in the MR-Linac Consortium and Elekta expects to see a pipeline of clinical trials exploring both concepts.
The MRIdian has a 0.35T MR scanner. While low-field MR systems are not typically used in diagnostic radiology, the lower field strength is advantageous in RT because it avoids the influence of a strong magnetic field on the radiation dose distribution. With a lower-field MR system, users can avoid most of the unavoidable interaction of the strong magnetic field’s influence on the radiation dose distribution, explains Martin Fuss, MD, chief medical officer of ViewRay and an oncologist with Radiation Oncology Specialists PC in Portland, OR. Rather than use an existing diagnostic MR scanner, ViewRay designed an MRI magnet and integrated it with a linac for the explicit purpose of delivering radiation therapy. It’s a compromise, Dr. Fuss adds, between good image quality and maintaining the capabilities of the MRI scanner to enable imaging while the radiation beam is on.
Of the 45 installed and operational MRIdian systems treating patients globally, 18 are in the US. More than 14,300 patients have been treated on MRIdian with clinically reported outcomes in over 3,200 patients. In 2020 in the US, 87% of all treatment courses on MRIdian systems were delivered by stereotactic body radiation therapy (SBRT). Nearly 1 in 4 (23%) of these patients received treatment for pancreatic cancer with 96% of the SBRT fractions adjusted daily using on-table or online adaptive replanning. By comparison, only 12% of plans for treating prostate cancer (18% of patients) are adapted on the day of treatment. For liver lesions (16% of patients) and lung tumors (10% of patients), 41% and 33%, respectively, of all delivered fractions were adapted with the patient on the table.
Routinely Delivering Ablative Doses
The use of ablative doses with MRgRT has become fairly routine at Miami Cancer Institute. Dr. Chuong says patients with no other options after enduring many lines of systemic therapy are now disease free thanks to the higher doses enabled by MRgRT.6
Dr. Chuong and colleagues published a retrospective analysis of 35 pancreatic cancer patients, most with locally advanced disease, who were treated with 5-fraction stereotactic MR-guided adaptive radiation therapy (SMART) in consecutive days. Approximately 91% received induction chemotherapy for several months prior to SMART.7
“The 2-year median survival control and progression-free survival numbers are all significantly higher than historical control and approaching the range of what you would expect from surgical resection,” says Dr. Chuong.
Dr. Chuong and his colleagues are also evaluating single-fraction ablative radiation therapy for patients with oligometastatic disease in the SMART ONE trial.8 This prospective trial aims to confirm the feasibility of using MRI guidance to complete ablative treatment to tumors in the chest, abdomen, and pelvis in only 1 fraction vs several, which would be especially beneficial for treatment of multiple oligometastases.
“MR guidance offers significant benefits for treating some oligometastatic tumors, especially given that the randomized data showing the addition of SBRT to systemic therapy improves overall survival,” Dr. Chuong says. Considering that oligometastatic lesions may be near radiosensitive organs (eg, the bowel) that are intolerant of high doses, he believes MRgRT will help widen the population cohort eligible for ablative radiation therapy and provide safe, effective outcomes.
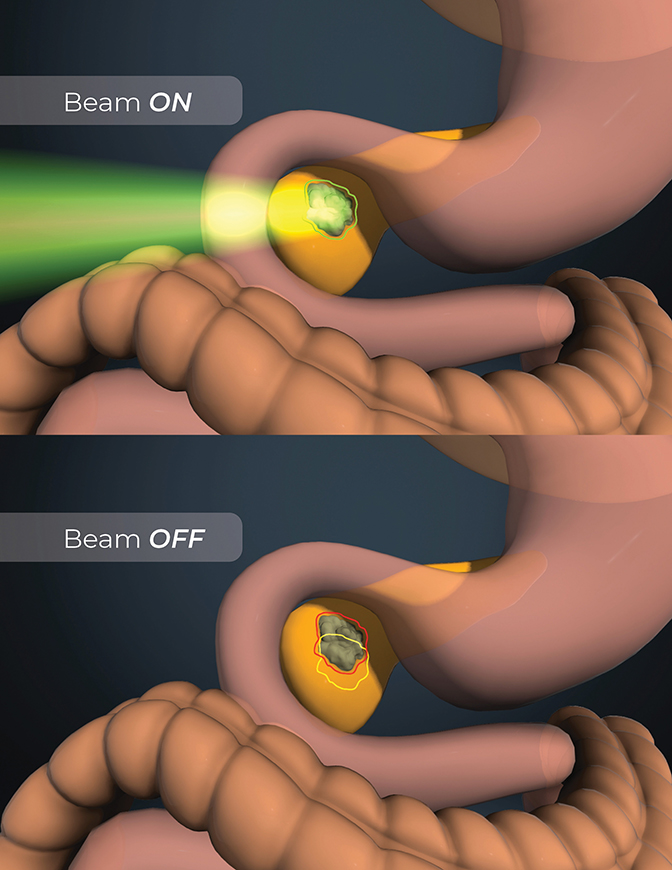
An important differentiator of MRIdian is the ability to perform on-table adaptive radiation therapy along with the system’s real-time, soft-tissue tracking and automated beam gating features. “With the integration of an MR scanner into a radiation dose delivery device, clinicians now have the ability to keep looking at the target while they deliver the dose,” says Dr. Fuss. “That is critical, because for the first time we don’t have to assume that the target resides in the right location relative to the damaging radiation beam. We can confirm that location multiple times a second.”
This capability changes the decades-old paradigm that nearby normal tissues limit the ability to deliver dose. “Now, we are able to break that paradigm and stay away from normal tissue during treatment,” he says. “We can now control depositing damaging doses to tumor tissue without causing toxicities to nearby organs at risk. This is only possible due to the integrated soft-tissue tracking and associated automated beam gating to either switch the radiation beam on or pause it.” (Figure 3)
Although the capability to perform on-table adaptive RT requires direct physician and physicist involvement during treatment, it has enabled Dr. Chuong to safely prescribe ablative doses. Over time, Miami Cancer Institute has become more efficient with this new workflow, which initially could take up to 90 minutes but now is routinely completed in 60 minutes. To help address the need for replanning, Miami Cancer Institute has extensively trained senior therapists on anatomy to aid the contouring process, which is then reviewed by the physician.
In pancreatic cancer patients treated with MRgRT, nearly all have had their plans reoptimized while on the table because critical organ constraints would otherwise have been exceeded if using the original treatment plan. “The ability to adapt is important,” Dr. Chuong explains. “Even if you don’t adapt, the confidence that the dose is safe to deliver and the understanding of how those changes in anatomy can affect the plan are key.”
For example, he prescribed 50 Gy/5 fractions to a metastatic lymph node next to the brachial plexus because he had the ability to adapt and contour the dose each day based on the shoulder position. “If I was treating this patient on any other machine, I almost certainly would not have prescribed that dose because I wouldn’t have had the certainty and confidence to do so. That patient had a complete response and remains disease free 3 years later,” he says.
Advancing MRgRT
Dr. Sahgal and co-authors recently demonstrated the feasibility of using a 1.5T MR-linac for in vivo chemical exchange saturation transfer (CEST), a type of metabolic imaging, of central nervous system tumors during radiation therapy to monitor treatment response. Changes were observed in individual patients over time, including between treatment fractions, as well as differences between high- and low-grade tumors. The authors also reported significant CEST signal contrast between the tumor and contralateral normal-appearing white matter (cNAWM) regions.9
“We are getting to the point of incorporating functional metabolic imaging beyond diffusion,” says Dr. Sahgal. “Metabolic imaging, in addition to high-quality diffusion imaging, will allow us to really drill down to the cellular level of what is happening in these tumors [during treatment] to create a biologically based adaptive MRgRT paradigm.”
Currently, hundreds of technical or clinical projects on Elekta Unity are in progress. These include the Hermes study at the Royal Marsden NHS Foundation Trust investigating the safety of using SBRT to deliver 2 fractions over 8 days vs 5 fractions over 10 days for localized prostate cancer,10 and the MR Adaptor at MD Anderson to compare the use of weekly adapted intensity-modulated radiation therapy (IMRT) to standard nonadapted IMRT in patients with low-risk human-papilloma-virus-positive oropharyngeal cancer.11
In addition to implementing MR safety policies and procedures, clinicians can expect a learning curve regarding tracking structures or boundaries with MR instead of CT images. To assist, ViewRay and Miami Cancer Institute are launching an advanced user group and training course for MRIdian focused on workflow, efficiency, and improvements for on-table adaptive therapy.
According to Dr. Fuss, two areas where ViewRay is enhancing the system are in improving workflow and developing site-specific coils. The company is also working with clinical partners to further extend functional imaging capabilities, such as recently licensing diffusion-weighted imaging capabilities from UCLA that enable b values up to 800.
“Many of the current system enhancements were first brought to us or had been requested by our clinical partners,” says Dr. Fuss. “For example, the team at UCLA first demonstrated the ability to acquire DWI data on the MRIdian. The associated clinical data was so compelling, that we licensed the sequence and made it available to our install base.”
Elekta will be adding enhancements to its MR protocol library with 7 new sequences intended to address specific clinical problems, says Dr. Christodouleas. These include an 18-second T2 with breath hold for thoracic imaging that leverages Philips Healthcare’s Compressed SENSE acceleration technique. The next software upgrade will also support gating functionality for motion management in 3 dimensions.
References
- Chuong M. Long-term outcomes of MR-guided SABR & on-table adaptive replanning for unresectable pancreas cancer. ESTRO 2021. Presentation Number: OC-0415. Accessed August 31, 2021. https://www.estro.org/Congresses/ESTRO-2021/502/profferedpapers25-uppergi/3760/long-termoutcomesofmr-guidedsabr-on-tableadaptiver
- NIH U.S. National Library of Medicine. The MOMENTUM Study: The Multiple Outcome Evaluation of Radiation Therapy Using the MR-Linac Study (MOMENTUM). Accessed August 30, 2021. https://clinicaltrials.gov/ct2/show/NCT04075305
- Stewart J, Sahgal A, Lee Y, et al. Quantitating interfraction target dynamics during concurrent chemoradiation for glioblastoma: a prospective serial imaging study. Int J Radiat Oncol Biol Phys. 2021 Mar 1;109(3):736-746. doi:10.1016/j.ijrobp.2020.10.002
- UNIty-Based MR-Linac Guided AdapTive RadiothErapy for High GraDe Glioma: a phase 2 trial (UNITED). Accessed August 30, 2021. https://clinicaltrials.gov/ct2/show/NCT04726397
- Lagendijk JJ, Raaymakers BW, Raaijmakers AJ, et al. MRI/linac integration. Radiother Oncol. 2008 Jan;86(1):25-9. doi:10.1016/j.radonc.2007.10.034
- Chuong M, Alvarez D, Romaguera T, et al. (2020). Case report of ablative magnetic resonance-guided stereotactic body radiation therapy for oligometastatic mesenteric lymph nodes from bladder cancer. Ther Radiol Oncol. 2020;4:20.
- Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-fraction stereotactic magnetic resonance-guided radiation therapy with on-table adaptive replanning and elective nodal irradiation for inoperable pancreas cancer. Pract Radiat Oncol. 2021 Mar-Apr;11(2):134-147. doi: 10.1016/j.prro.2020.09.005. Epub 2020 Sep 16. Erratum in: Pract Radiat Oncol. 2021;11(3):e354.
- NIH U.S. National Library of Medicine. Stereotactic MRI-guided Adaptive Radiation Therapy (SMART) in One Fraction (SMART ONE). Accessed August 30, 2021. https://clinicaltrials.gov/ct2/show/NCT04939246?term=SMART&cond=cancer&cntry=US&state=US%3AFL&draw=2&rank=5
- Chan RW, Lawrence LSP, Oglesby RT, et al. Chemical exchange saturation transfer MRI in central nervous system tumors on a 1.5 T MR-Linac. Radiother Oncol. 2021;162:140-149. doi:10.1016/j.radonc.2021.07.010. Epub ahead of print.
- NIH U.S. National Library of Medicine. Hypofractionated Expedited Radiotherapy for Men With localisEd proState Cancer (HERMES). Accessed August 30, 2021. https://clinicaltrials.gov/ct2/show/NCT04595019
- NIH U.S. National Library of Medicine. Trial of Magnetic Resonance Imaging Guided Radiotherapy Dose Adaptation in Human Papilloma Virus Positive Oropharyngeal Cancer. Accessed August 30, 2021. https://www.clinicaltrials.gov/ct2/show/NCT03224000
Citation
MB M. Magnetic Appeal: Changing Treatment Paradigms with MR-Guided Radiation Therapy. Appl Radiat Oncol. 2021;(3):56-59.
October 5, 2021