Lung cancer radiation therapy: Defining optimal evidence-based treatment approaches
Images


As the second most common cancer in the United States with an estimated incidence of more than 220,000 cases per year, lung cancer remains the leading cause of cancer mortality with 158,000 deaths annually.1 However, lung cancer is not a homogeneous disease process, but rather a complex entity that goes far beyond traditional dichotomies of small cell (SCLC) and non-small cell lung cancer (NSCLC). Recent studies have highlighted this fact, demonstrating that even within histologic subsets of NSCLC, different treatment paradigms may be required based on tumor biology and tumor genetics.2,3 Further, treatment techniques for surgery, radiation therapy, and systemic therapy have evolved as well, providing physicians with new modalities and treatment options for patients regardless of stage. As such, clinicians treating lung cancer are tasked with constantly re-evaluating emerging data and techniques to offer their patients evidence-based treatment options. Such innovations and paradigm shifts have been particularly evident in radiation oncology, where significant changes to treatment indications, techniques, and principles have occurred over the past decade. Therefore, the purpose of this review is to provide clinicians with a framework to make decisions regarding radiation therapy in lung cancer based on recent data as well as recent guidelines and treatment pathways.
Discussion
Non-Small Cell Lung Cancer
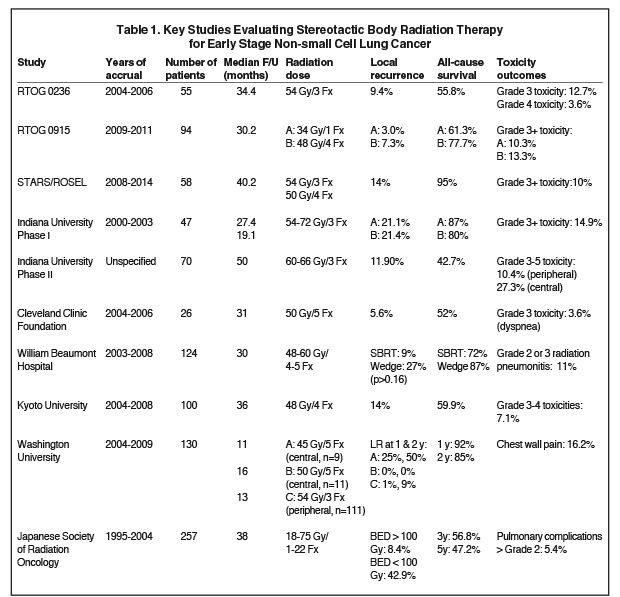
In patients with early stage NSCLC (T1-2N0), the standard of care for many years has been surgery with consideration for adjuvant chemotherapy.4 However, many patients are deemed inoperable due to inadequate pulmonary function or other medical comorbidities, while some patients refuse surgery. Traditionally, these patients were offered definitive standard fractionation radiation therapy, which was associated with poor outcomes, even with dose escalation.5-7 With the advent of advanced treatment planning and delivery systems in conjunction with image guidance, stereotactic body radiation therapy (SBRT) has emerged, allowing for the delivery of large doses per fraction with highly conformal dose distributions and real-time online image verification. One of the initial series evaluating SBRT came from Indiana University where an initial phase I dose escalation study was followed by a phase II study of medically inoperable patients (< 7 cm) with early stage (T1-2N0) NSCLC. Patients were treated with 60-66 Gy in 3 fractions and with 4-year follow-up, local control was 88% and cause-specific survival was 82%.8,9 Importantly, however, grade 3 or greater toxicity was noted to be higher with central tumors (27% vs. 10%).10 These promising initial findings were confirmed by additional series.11-13 RTOG (Radiation Therapy Oncology Group) 0236 was a multi-institutional phase II trial of 55 patients (T1-2N0, < 5 cm, peripheral location, nonsurgical candidates) in which patients received SBRT (54 Gy/3 fractions); with 3-year follow-up, tumor control was 98% with a 91% rate of local (lobar) control and 87% locoregional control. Grade 3 toxicities were seen in 13% of patients with 4% of patients developing grade 4 toxicities and no grade 5 toxicities reported.12 Table 1 summarizes key studies evaluating SBRT.8-18
One of the greatest challenges facing clinicians is deciding on patient eligibility for SBRT as well as appropriate dose and fractionation schedules.18-21 Table 2 presents a summary of inclusion criteria for peripheral and central tumors as well as evidence-based fractionation schemes. An additional question facing clinicians is the role of SBRT in operable patients, as initial studies have suggested comparable outcomes.12 Additionally, data from William Beaumont Hospital suggested lower rates of local recurrence with SBRT and comparable cause-specific survival as compared to wedge resection, while a pooled analysis of the Stereotactic Ablative Radiotherapy (SABR) in Stage I Non-small Cell Lung Cancer Patients Who Can Undergo Lobectomy (STARS) and Trial of Either Surgery or Stereotactic Radiotherapy for Early Stage (IA) Lung Cancer (ROSEL) phase 3 trials evaluating SBRT in operable patients found improved survival with SBRT compared to surgery at 3 years.16,17 Similarly, a pooled analysis from Crabtree et al found that when using propensity analyses, SBRT was associated with similar rates of local control and cancer-specific survival compared with surgery in patients with stage I disease.22 RTOG 0618 was a phase II trial evaluating medically operable patients (T1-2N0, < 5 cm, noncentral tumors) treated with SBRT (60 Gy/3 fractions) with outcomes expected in the next few years.19
For patients with locally advanced resectable NSCLC, neoadjuvant chemoradiation can be considered. Eligibility includes the patient being a surgical candidate with respect to medical comorbidities and pulmonary function (FEV1 > 2 L, predicted postoperative FEV > 1.2 L) with limited N2 nodal disease, and without N3 or T4 disease.23 Patients typically receive 45-50 Gy with concurrent chemotherapy with restaging 2-4 weeks later, followed by surgery. The Intergroup 0139 trial compared this approach to definitive chemoradiation and found no difference in median or overall survival at 5 years; however, improvements in progression-free survival with neoadjuvant therapy were noted, as was improved survival for the subset of patients undergoing lobectomy.23 An increase in treatment-related deaths was noted with neoadjuvant treatment followed by surgery (primarily in the pneumonectomy cohort), although rates of grade 3-4 esophagitis were reduced compared to definitive chemoradiation.23
For patients with locally advanced unresectable NSCLC who are fit for definitive therapy, chemoradiation is the standard of care.4 This represents an evolution of treatment paradigms from radiation alone to sequential chemotherapy and radiation therapy to concurrent therapy.4,24-26 The basis for this recommendation is several studies that have demonstrated a benefit in survival with concurrent therapy, as compared to sequential therapy.24-26 Further, a pooled analysis comparing sequential and concurrent therapies found a 4.5% improved overall survival at 5 years with concurrent therapy, as well as reduced locoregional recurrences.27 However, the tradeoff for this survival benefit was an increase in acute grade 3-4 esophageal toxicity (18% vs. 4%).27 As for radiation dose, preliminary data evaluating dose escalation were promising.28 However, RTOG 0617, a 4-arm phase III trial, found no benefit to dose escalation (74 Gy vs. 60 Gy) with a significant improvement in overall survival noted with 60 Gy, and reduced quality of life with dose escalation.29,30 At this time, the role of dose escalation in patients receiving concurrent therapy is limited, but for patients unable to receive chemotherapy, there are data to support dose escalation when meeting organ-at-risk dose-volume constraints.6,7,31
While the role of postoperative radiation therapy (PORT) is often considered controversial, patients should be evaluated for adjuvant radiation therapy when there are positive margins or N2 nodal involvement (and potentially N1 patients not receiving chemotherapy).4,32 For patients with N2 disease, while older data support a benefit to PORT, recent subset data from the ANITA (Adjuvant Navelbine International Trialist Association) trial as well as a SEER (Surveillance, Epidemiology, and End Results) analysis have also demonstrated improved survival with the addition of PORT in N2 patients, which is reflected in evidence-based guidelines.4 Regarding sequencing, adjuvant chemotherapy is typically followed by PORT. However, in patients with positive margins, consideration for adjuvant chemoradiation should be made.4,33-35 With respect to adjuvant chemoradiation, RTOG 9705 was a phase II trial of 88 patients (stage II/IIIA disease following surgery), with patients receiving concurrent chemotherapy (paclitaxel/carboplatin) and radiation (50.4 Gy/28 fractions, 10.8 Gy boost for nodal ECE or T3 disease). With 5-year follow-up, local failure was 15% and median survival 57 months with an acceptable toxicity profile.36
Small Cell Lung Cancer
Radiation therapy has represented a standard approach to managing limited stage SCLC for several decades with the MRC trial from the 1960s demonstrating improved survival with definitive radiation as compared to surgery in operable patients.37,38 Further, while chemotherapy remains a mainstay of treatment for SCLC, two meta-analyses have demonstrated improved survival with the addition of radiation to systemic therapy.39,40 More recently, concurrent chemoradiation has become the standard-of-care approach, with radiation traditionally combined with cisplatin and etoposide.38,41 The Intergroup 0096 trial randomized 417 patients to 45 Gy/25 fractions or 45 Gy/30 fractions (twice daily) with both arms receiving cisplatin/etoposide, and radiation fields that included the bilateral mediastinum and ipsilateral hilum. At 8 years, hyperfractionation was associated with improved 5-year overall survival (26% vs. 16%), with increased rates of esophagitis (27% vs. 11%) and a trend for improved local control; however, a criticism of this trial is that the two arms did not receive biologically equivalent doses, biasing the trial toward hyperfractionation.42 Dose escalation has been evaluated, as have alternative schedules. One such regimen, which uses the concomitant boost approach (initially 1 fraction/day, then twice daily to finish within 5 weeks) was evaluated in RTOG 9712 with concurrent chemotherapy with a maximum tolerated dose of 61.2 Gy.43 Subsequently, this regimen was evaluated on RTOG 0239 and found to have a 2-year survival of 37%, with an 18% rate of severe esophagitis and 3% treatment-related deaths.44 Similarly, studies evaluating the maximum tolerated dose with once daily radiation therapy reached a dose of 70 Gy.45,46 As such, the CALGB (Cancer and Leukemia Group B) 30610/RTOG 0538 trial is comparing 45 Gy/30 fractions twice daily with 61.2 Gy concomitant boost, and 70 Gy once daily with results expected in the years to come; however, the 61.2 Gy was closed leaving the hyperfractionation and the 70 Gy arms open.47 As for the timing of chemoradiation, while individual studies have been mixed, a meta-analysis has demonstrated an improvement with early thoracic radiation therapy within 30 days of starting chemotherapy.38,47-50
The role of thoracic radiation therapy in patients with extensive stage SCLC remains controversial. Jeremic et al presented a randomized study of 210 patients with extensive-stage SCLC who had a complete distant response and a complete/partial response locally following chemotherapy (cisplatin/etoposide). Patients were randomized to further chemotherapy without radiation or chemoradiation (54 Gy/36 fractions twice daily with carboplatin/etoposide). The study found that median survival (17 months vs. 11 months) and 5-year survival (9% vs. 4%) improved with thoracic radiation therapy.51 A larger multi-institutional randomized study included 498 patients with a response to chemotherapy, with patients receiving either thoracic radiation therapy (30 Gy/10 fractions) or no thoracic radiation with all receiving PCI (prophylactic cranial irradiation). With 2-year follow-up, thoracic radiation therapy improved 2-year survival (13% vs. 3%) with improved progression-free survival (24% vs. 7%) also noted.52 However, recently RTOG 0937 was published; this was a randomized phase II trial in which patients with extensive stage SCLC (1-4 metastatic lesions, no brain metastases) who had a partial/complete response to chemotherapy were randomized to consolidative radiation therapy to the thorax and metastatic sites to a dose of 45 Gy/15 fractions (allowed to treat 30-40 Gy/10 fractions if necessary). A total of 97 patients were enrolled and with short follow-up, consolidative radiation therapy was found to delay progression with no improvement in survival noted.53 At this time, the role of thoracic/consolidative radiation therapy remains unclear with further data required; however, its use is supported by evidence-based guidelines.38
PCI represents a standard-of-care treatment approach for patients with limited and extensive stage SCLC.38 For patients with limited stage SCLC, several studies have confirmed a reduction in brain metastases with PCI in patients with a complete response to therapy, although no survival benefit was noted.54,55 However, a meta-analysis from Auperin et al evaluated 7 randomized trials (987 patients) and found that that PCI improved OS at three years (21% vs. 15%) for patients with a complete response to therapy.56 Additionally, larger radiation doses were associated with a greater reduction in brain metastases without survival benefit. For patients with extensive-stage SCLC, the EORTC (European Organization for Research and Treatment of Cancer) 08993 trial randomized 286 patients with extensive-stage SCLC who had any response to 4-6 cycles of chemotherapy to PCI (20 Gy/5 fractions- 30 Gy/12 fractions) or no PCI. At 1 year, PCI reduced the rates of symptomatic brain metastases (15% vs. 40%) and, more importantly, improved survival (27% vs. 13%), although neuroimaging was not required beforehand.57 Regarding dose, the standard PCI dose remains 25 Gy in 10 fractions, although alternatives have been used, including 20 Gy/5 fractions in 60% of cases in the EORTC study.38,57 At this time, data does not support dose escalation for PCI. RTOG 0212 randomized 720 patients with limited stage SCLC who had complete response to chemoradiation to PCI with either 25 Gy/10 fractions or a higher dose (36 Gy/18 fractions or 36 Gy/24 fractions BID), with all patients receiving baseline neuroimaging. Results from this study demonstrated no difference in the incidence of brain metastases between regimens with improved survival with the standard PCI dose (42% vs. 37%, p = 0.05) at 2 years.58 Concerns, however, exist regarding the potential neurotoxicity associated with PCI. Health-related quality-of-life studies from the EORTC trial demonstrated a negative impact with PCI (primarily fatigue and hair loss) with limited impact on global health status.59 Strategies emerging to reduce PCI-related toxicity include hippocampal sparing, which is being evaluated on NRG-CC003, as well as memantine.60,61
Radiation Therapy Techniques
Safe and effective SBRT requires modern treatment planning and delivery techniques. Patients treated with SBRT should undergo CT simulation with respiratory motion management (4D-CT, abdominal compression, and/or gating) and immobilization. Standard volumes include a GTV (gross tumor volume, as defined on CT using lung windows), which is equal to the clinical target volume (CTV). Planning tumor volume (PTV) margins can vary depending on image guidance techniques, with RTOG 0618 using a 5-mm radial and 10-mm longitudinal expansion.19-21 Planning can be performed using coplanar and noncoplanar beam arrangements with typically 10 or more beams; alternatively, rotational/arc-based techniques (eg, volumetric-modulated arc therapy) can be used.19-21,62 An important consideration in SBRT planning is target volume coverage and normal tissue constraints. When reviewing target coverage, the following should be evaluated: 1) normalization to the center of mass of the PTV, 2) isodose line of 60-90% encompassing 95% of the PTV (such that 99% of the PTV receives at least 90% of prescription dose), and 3) restriction of where high dose is delivered (limit dose > 105% of prescription to PTV, all tissue outside PTV receiving > 105% of prescription should be < 15% of PTV volume) while maintaining conformality.19-21 As for normal tissue constraints, RTOG 0618 and RTOG 0915 provide constraints for SBRT of peripheral lesions, while RTOG 0813 provides constraints for central tumors; published constraints are available as well (Table 3).19-21,63,64
Patients treated with definitive radiation therapy for NSCLC and SCLC should undergo CT simulation with respiratory motion management (4D-CT, breath-hold, or active breathing control [ABC]) and immobilization. For NSCLC, the GTV is defined as the primary tumor and involved nodes (can use PET scan and other studies). The CTV is defined as an expansion for subclinical involvement, typically from 5-10 mm, with RTOG 1308 using an 8-mm expansion, excluding uninvolved organs.65 Accounting for respiratory motion is the internal tumor volume (ITV), which can be done by creating a CTV on the iGTV or by creating a union of CTVs. PTV margin is typically 5 mm.65 One question concerning CTV volume centers on the role of elective nodal irradiation (ENI). In NSCLC, data from Memorial Sloan Kettering Cancer Center identified a 6% rate of elective nodal failure when omitting ENI, confirmed by a randomized study from China.66,67 However, a report from the International Atomic Energy Agency supports a more nuanced approach rather than completely omitting ENI, with potential utilization of ENI based on factors including stage and tumor location.68 For treatment planning techniques, both 3-dimensional conformal radiation therapy (3D-CRT) and intensity-modulated radiation therapy (IMRT) can be used.29 While IMRT has been shown to improve some dosimetric parameters when compared to 3D-CRT, clinical data comparing techniques are limited.69,70 A recent secondary analysis of RTOG 0617, however, found that IMRT reduced the rates of severe pneumonitis in patients receiving chemoradiation, while a recent population-based study found improved survival with IMRT for T3/4 tumors.71,72
As with NSCLC, treatment techniques in SCLC have evolved over several decades. Classically, the field design from the Intergroup trial included the primary tumor as well as the ipsilateral hilum and bilateral mediastinum, extending 5 cm below the carina or to the ipsilateral hilum (whichever was lower) with the clinical volume expanded 1-1.5 cm.42 Field arrangements included the use of oblique off-cord fields for the afternoon fraction in weeks 2 and 3. Since this study, changes have occurred concerning target volumes and treatment planning. As noted above, traditional SCLC volumes included elective nodal irradiation. However, data have emerged demonstrating low rates of elective nodal failure (< 5%), particularly when using positron emission tomography (PET) scans as part of treatment planning.73-75 As such, current trials have moved away from elective nodal coverage and treat involved nodes only.
Another important question is whether target volumes should include prechemotherapy disease or postinduction volumes in patients not receiving radiation in conjunction with the first cycle of chemotherapy. Currently, although data remains limited, the use of postchemotherapy volumes is supported by data demonstrating no difference in the rates of marginal failures with the use of postinduction volumes.76 Regarding the current standard of care, CALGB 30610 mandates CT-based planning with respiratory management strongly encouraged, and treatment planning with either 3D-CRT or IMRT. Target volumes include the GTV (as defined by physical exam, CT, PET and/or MRI). The ITV incorporates tumor motion during the respiratory cycle, while the CTV expansion allows for occult disease without elective nodal irradiation.47 When delivering PCI, the standard field arrangement is opposed lateral fields covering the entire cranial contents. However, with the use of hippocampal sparing, new planning techniques are available.60
Conclusions
Radiation therapy represents a standard treatment option in the management of lung cancer, from early stage NSCLCs treated with SBRT to ES-SCLC, which can be treated with PCI and thoracic radiation therapy. Treatment techniques continue to evolve to help maximize the therapeutic ratio and improve not only clinical outcomes, but also toxicity profiles and quality of life for patients receiving treatment.
REFERENCES
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
- Yoshimura N, Kudoh S, Mitsuoka S, et al. Phase II study of combination regimen of gefitinib and pemetrexed as first-line treatment in patients with advanced non-small cell lung cancer harboring a sensitive EGFR mutation. Lung Cancer. 2015;90:65-70.
- Karachaliou N, Rosell R, Morales-Espinosa D, et al. Systemic treatment in EGFR-ALK NSCLC patients: second line therapy and beyond. Expert Rev Anticancer Ther. 2014;14:807-815.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 4.2016. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. (Login required.) Accessed August 20, 2016.
- Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest. 2005;128:1461-1467.
- Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311; a phase I-II dose escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318-328.
- Sura S, Yorke E, Jackson A, et al. High-dose radiotherapy for the treatment of inoperable non-small cell lung cancer. Cancer J. 2007;13:238-242.
- McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys. 2005;63:1010-1015.
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677-682.
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833-4839.
- Videtic GM, Stephans K, Reddy C, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: excellent local control. Int J Radiat Oncol Biol Phys. 2010;77:344-349.
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for state I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94-100.
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427-1431.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070-1076.
- Olsen JR, Robinson CG, El Naga I, et al. Dose-response for stereotctic body radiotherapy in early-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e299-303.
- Grills IS, Mangona VS< Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28:928-935.
- Chang JY, Senan S, Paul MA. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630-637.
- Videtic GM, Hu C, Singh AK, et al. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys. 2015;93:757-764.
- RTOG 0618: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Operable Stage I/II Non-Small Cell Lung Cancer. Available at: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0618. Accessed August 20, 2016.
- RTOG 0915: A Randomized Phase II Study Comparing 2 Stereotactic Body Radiation Therapy (SBRT) Schedules for Medically Inoperable Patients with Stage I Peripheral Non-Small Cell Lung Cancer. Available at: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0915. Accessed August 20, 2016.
- RTOG 0813: Seamless Phase I/II Study of Stereotactic Lung Radiotherapy (SBRT) for Early Stage, Centrally Located, Non-Small Cell Lung Cancer (NSCLC) in Medically Inoperable Patients. Available at: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0813. Accessed August 20, 2016.
- Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg. 2014;147:1183-1191.
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomized controlled trial. Lancet. 2009;374:379-386.
- Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88:1210-1215.
- Furuse K, Fukoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692-2699.
- Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452-1460.
- Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181-90.
- Bepler G, Dilling TJ, Wagner H, et al. Phase II trial of induction gemcitabine and carboplatin followed by conformal thoracic radiation to 74 Gy with weekly paclitaxel and carboplatin in unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2011;6:553-558.
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187-199.
- Movsas B, Hu C, Sloan J, et al. Quality of Life Analysis of a Radiation Dose-Escalation Study of Patients With Non-Small-Cell Lung Cancer: A Secondary Analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol 2016;2:359-367.
- Kong FM, ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: results of radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324-333.
- PORT Meta-analysis Trialists Group. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2005;18:CD002142.
- Sawyer TE, Bonner JA, Gould PM, et al. The impact of surgical adjuvant thoracic radiation therapy for patients with nonsmall cell lung carcinoma with ipsilateral mediastinal lymph node involvement. Cancer. 1997;80:1399-1408.
- Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24:2998-3006.
- Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the Adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72:695-701.
- Bradley JD, Paulus R, Graham MV, et al. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: promising long-term results of the Radiation Therapy Oncology Group--RTOG 9705. J Clin Oncol. 2005;23:3480-3487.
- Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet. 1973;2:63-65.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer. Version 1.2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed August 20, 2016.
- Pignon JP, Arriagad R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618-1624.
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890-895.
- Thomas CR, Giroux DJ, Janaki LM, et al. Ten-year follow-up of Southwest Oncology Group 8269: a phase II trial of concomitant cisplatin-etoposide and daily thoracic radiotherapy in limited small-cell lung cancer. Lung Cancer. 2001;33:213-219.
- Turrisi AT, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265-271.
- Komaki R, Swann RS, Ettinger DS, et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer: report of Radiation Therapy Oncology Group (RTOG) protocol 97-112.
- Komaki R, Paulus R, Ettinger DS, et al. A phase II study of accelerated high-dose thoracic radiation therapy (AHTRT) with concurrent chemotherapy for limited small cell lung cancer: RTOG 0239. J Clin Oncol. 2009;27:15s.
- Hoic NC, Herndon JE, Rosenman J, et al. Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol. 1998;16:3528-3536.
- Bogart JA, Herndon JE, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group study 39808. Int J Radiat Oncol Biol Phys. 2004;59:460-468.
- CALGB 30610/RTOG 0538: CALGB 30610/Endorsed Study: Phase III Comparison of Thoracic Radiotherapy Regimens in Patients with Limited Small Cell Lung Cancer Also Receiving Cisplatin and Etoposide. Available at: https://www.rtog.org/ClinicalTrials/ProtocolTable.aspx. Accessed August 20, 2016.
- Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336-344.
- Jeremic B, Milicic B. Influence of interfraction interval on local tumor control with limited-disease small-cell lung cancer treated with radiochemotherapy. Int J Radiat Oncol Biol Phys. 2007;68:426-432.
- Pijls-Johannesma M, De Ruysscher D, Vansttenkiste J, et al. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev. 2007;33:461-473.
- Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol. 1999;17:2092-2099.
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer a phase 3 randomised controlled trial. Lancet. 2015;385:36-42.
- Gore EM, Hu C, Sun A, et al. NRG Oncology/RTOG 0937: Randomized phase 2 study comparing prophylactic cranial irradiation (PCI) alone to PCI and consolidative extracranial irradiation for extensive disease small cell lung cancer (ED-SCLC). Int J Radiat Oncol Biol Phys. 2016;94:s5.
- Laplanche A, Monnet I, Santos-Miranda JA, et al. Controlled clinical trial of prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Lung Cancer. 1998;21:193-201.
- Gregor A, Cull A, Stephens RJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer. 1997;33:1752-1758.
- Auperin A, Arriagad R, Pignon JP. Prophylactic cranial irradiation for patients with small-cell lung cancer with complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476-484.
- Slotman B, Faivre-Finn C, Kramer C, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664-672.
- Le Pechoux C, Dunant A, Wilfson A, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, IFCT 99-01): a randomised clinical trial. Lancet Oncol. 2009;10:467-474.
- Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an internal phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol. 2009;27:78-84
- NRG-CC003: NRG-CC003: A Randomized Phase II/III Trial of Prophylactic Cranial Irradiation with or without Hippocampal Avoidance for Small Cell Lung Cancer. Available at: https://www.nrgoncology.org/Clinical-Trials/NRG-CC003. Accessed August 20, 2016.
- Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429-1437.
- Dickey M, Roa W, Drudge S, et al A planning comparison of 3-dimensional conformal multiple static field, conformal arc, and volumetric modulated arc therapy for the delivery of stereotactic body radiotherapy for early stage lung cancer. Med Dosim. 2015;40:347-351.
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-19.
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:348-353.
- RTOG 1308: Phase III Randomized Trial Comparing Overall Survival After Photon Versus Proton Chemoradiotherapy for Inoperable Stage II-IIIB NSCLC. Available at: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1308. Accessed August 20, 2016.
- Rosenzweig KE, Sura S, Jackson A. Involved-field radiation therapy for inoperable non small-cell lung cancer. J Clin Oncol. 2007;25:5557-5561.
- Yuan S, Sun X, Li M, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol. 2007;30:239-244.
- Belderbos JS, Kepka L, Spring Kong FM, et al. Report from the International Atomic Energy Agency (IAEA) consultants’ meeting on elective nodal irradiation in lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:335-342.
- Liu HH, Wang X, Dong L, et al. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1268-1279.
- Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94-102.
- Chun SG, Hu C, Choy H, et al. Comparison of 3-D conformal and intensity modulated radiation therapy outcomes for locally advanced non-small cell lung cancer in NRG Oncology/RTOG 0617. Int J Radiat Oncol Biol Phys. 2015;93:S1-2.
- Jegadeesh N, Liu Y, Gillespie T, et al. Evaluating intensity-modulated radiation therapy in locally advanced non-small-cell lung cancer: results from the national cancer database. Clin Lung Cancer. 2016 Feb 2 [Epub ahead of print].
- Baas P, Belderbos JS, Senan S, et al. Concurrent chemotherapy (carboplatin, paclitaxel, etoposide) and involved-field radiotherapy in limited stage small cell lung cancer: a Dutch multicenter phase II study. Br J Cancer. 2006;94:625-630.
- De Ruysscher D, Bremer RH, Koppe F, et al. Omission of elective node irradiation on basis of CT-scans in patients with limited disease small cell lung cancer: a phase II trial. Radiother Oncol. 2006;80:307-312.
- Liengswangwong V, Bonner JA, Shaw EG, et al. Limited-stage small-cell lung cancer: patterns of intrathoracic recurrence and implications for thoracic radiotherapy. J Clin Oncol. 1994;12: 496-502.
- Bonner JA, Sloan JA, Shanahan, TG, et al. Phase III comparison of twice-daily split-course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. J Clin Oncol. 1999;17:2681-2691.
Citation
C S, T S, N K, S P, K S, G V. Lung cancer radiation therapy: Defining optimal evidence-based treatment approaches. Appl Radiat Oncol. 2016;(4):4-11.
December 12, 2016