Incidental nodal irradiation in locally advanced non-small cell lung cancer treated with involved-field IMRT
Images
Abstract
Background:
Studies using 3-dimensional conformal radiation therapy (3DCRT) show that elective nodal (EN) areas receive substantial incidental irradiation in non-small cell lung cancer (NSCLC) treated with involved-field radiation therapy (IFRT). Due to increasing use of intensity-modulated radiation therapy (IMRT), we performed a dosimetric analysis of 3DCRT vs. IMRT comparing EN incidental irradiation.
Material and Methods:
Twenty-three stage IIIA NSCLC patients treated with curative intent IMRT (median dose 72 Gy) were studied. Nodal stations 1-2, 3A, 3P, 4, 5, 6, and 7 were contoured. Comparative 3DCRT plans were generated. Mean dose, V40, V50 and V60 were compared for each station.
Results:
PTV V95 coverage was similar between 3DCRT and IMRT plans (p = 0.20). No significant differences in incidental irradiation were found except for contralateral 6 and ipsilateral station 5 nodes. For contralateral station 6, mean dose, V50 and V60 were less with IMRT than 3DCRT (43 Gy vs. 55 Gy, p = 0.01; 39% vs. 67%, p = 0.02; 14% vs. 58%, p = 0.002; respectively). IMRT also delivered less dose to ipsilateral station 5 compared to 3DCRT (mean 66 Gy vs. 71 Gy, p = 0.04). At a median follow up of 21 months, 6 patients (26%) had isolated locoregional recurrences, with only 1 patient (4%) having an isolated EN failure (station 5, supraclavicular) without intrathoracic progression.
Conclusions:
IFRT using IMRT delivers similar incidental irradiation doses as 3DCRT to EN stations and may be safely delivered without theoretical concern for increased EN failures. Caution should be noted when treating with IMRT if there is high risk for subclinical disease in levels 5 and 6.
The standard of care in locally advanced non-small cell lung cancer (NSCLC) includes concurrent chemotherapy and radiation therapy (RT).1,2 Major improvements in radiation technology have led to significant changes in radiation delivery for NSCLC, including 3D-conformal radiation treatment (3DCRT) and intensity-modulated radiation therapy (IMRT).These technologies have enabled the delivery of more conformal radiation to spare normal surrounding tissue.
Historically, elective nodal irradiation (ENI) was employed in locally advanced NSCLC to reduce regional failures in the mediastinal lymph node (LN) regions.3,4 More recently, treatment has evolved to involved-field radiation therapy (IFRT), in which EN regions are omitted to deliver higher doses of radiation to gross disease while decreasing subclinical treatment volumes to reduce toxicities to the esophagus, lung and heart.5-7 Multiple studies employing IFRT have demonstrated acceptable locoregional control rates, with 0% to 7% isolated nodal failures in EN stations outside initially involved LN regions, and most failures occurring in-field or distantly.7-10 A major contributor to low nodal failure rates may be the clinically meaningful incidental irradiation to EN stations delivered with IFRT.8,9,11,12 However, studies on EN failure patterns to date have primarily utilized 3DCRT, and it is unclear whether these data are applicable to more advanced modalities like IMRT.
IMRT is being increasingly used for NSCLC with the potential for more conformal radiation, with one study demonstrating an increase in IMRT from 2% in 2002 to 25% in 2009.6,13-17 Furthermore, recent cooperative group trials, including RTOG 061718 and RTOG 1308,19 have allowed IMRT for treatment. However, with the increasing use of IMRT, concerns have emerged that more conformal IFRT techniques may deliver less incidental irradiation to ENs and result in increased nodal failures, potentially compromising tumor control or patient survival. Due to the paucity of data to address this theoretical concern, we performed a dosimetric analysis of 3DCRT vs. IMRT treatment plans to compare incidental irradiation to thoracic nodal stations in locally advanced NSCLC.
Materials and Methods
Patient Selection
We studied 30 stage IIIA-IIIB NSCLC patients treated with curative intent IMRT at the University of Pennsylvania between 2009-2011 after approval from the institutional review board. All patients were staged upfront with PET/CT prior to treatment and treated with IFRT (defined below). Patients were predominantly treated on 2 prospective institutional protocols assessing dose escalation.
Inclusion criteria consisted of patients with histologically proven NSCLC, stages IIIA-IIIB, curative intent treatment, and radiation prescription doses ≥ 50 Gy. Stage IIIB patients with contralateral N3 disease were excluded since nearly all portions of the mediastinum would be comprehensively treated with either 3DCRT or IMRT given contralateral nodal disease. Most patients received chemotherapy, generally with concurrent carboplatin/paclitaxel, carboplatin/docetaxel, or cisplatin/etoposide/nelfinavir (an institutional protocol).
Radiation Treatment Planning
All patients were treated with step-and-shoot IMRT, and plans were created using Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA). Each patient underwent CT-based planning. All fields were treated daily. Patients were simulated supine with arms raised on a wing board using a 4-dimensional CT (4D CT). The gross tumor volume (GTV), clinical tumor volume (CTV), and planning tumor volume (PTV) were defined according to International Commission on Radiation Unit and Measurements (ICRU) 50. Nodal GTV was defined as biopsy-proven nodal disease, radiographic enlargement of LNs > 1 cm on CT, or fludeoxyglucose F18 positron emission tomography (18FDG-PET) positivity (SUV max ≥ 3.0).20 CTV expansion was 0.8-1.0 cm for primary GTV lesions and 0.3 cm for nodal GTV. An ITV expansion was created for target motion during the breathing cycle. The PTV was generated with a 0.5-cm margin around the ITV. Lung, esophagus, heart and spinal cord were contoured as organs at risk (OAR). OAR dose constraints were as follows: maximum spinal cord dose 50 Gy; total lung (lungs minus PTV) V20 < 35%, lung V5 < 60%, and mean lung dose < 20 Gy; heart V40 < 50%; and esophagus V55 < 30%.
IMRT plans were generated with 4-5 fixed fields, arranged primarily anterior/posterior with obliques to minimize lung dose. Fluence-based optimization with beamlet-based inverse planning was utilized. Each patient had a comparative 3DCRT treatment plan generated using Eclipse software. The 3DCRT beam arrangements were similar to their respective IMRT plans with anterior/posterior and opposed oblique fields with objectives to maximize tumor coverage while limiting lung exposure. The 3DCRT plans were optimized to have comparable PTV coverage while meeting dose constraints for lung, heart, esophagus and spinal cord. Seven patients with medially located gross disease near the spinal cord were excluded from dosimetric analysis as the cord dose constraint was exceeded, which precluded optimal PTV coverage in 3DCRT plans. Our final cohort consisted of 23 patients for dosimetric analysis.
Data Analysis
For the dosimetric analysis, LN stations 1-2, 3A, 3P, 4, 5, 6, and 7 were contoured according to the University of Michigan CT-based atlas of thoracic node regions.21 These nodal volumes were then truncated from the PTV to generate EN volumes. Dosimetric parameters were calculated for each EN station for both IMRT and 3DCRT plans (n = 46 plans). The mean dose (Gy) and V40, V50 and V60 (mean %) were calculated for each nodal station for plan comparisons. These volumetric dose levels were chosen as they are reflective of EN doses that may provide adequate microscopic disease control.9,11,22 Clinical outcomes, including locoregional recurrence, distant failures and survival, were assessed at longitudinal follow-up after treatment completion. Locoregional recurrences were defined as occurring in the lung or regional lymph nodes.
Statistical Analysis
Wilcoxon Rank sum test and 2-sample t tests were used to compare lung mean, lung V5, lung V20, cord max, PTV coverage V95, heart V40, esophagus V55, as well as mean radiation dose and V40, V50 and V60 at each nodal station. Chi-squared analysis was used to compare patient demographics, tumor stage and tumor laterality. Differences were considered statistically significant at p value < 0.05. Statistical analysis of data was performed using STATA data analysis software (Version 11 for Windows, College Station, TX).
Results
Patient and Tumor Characteristics
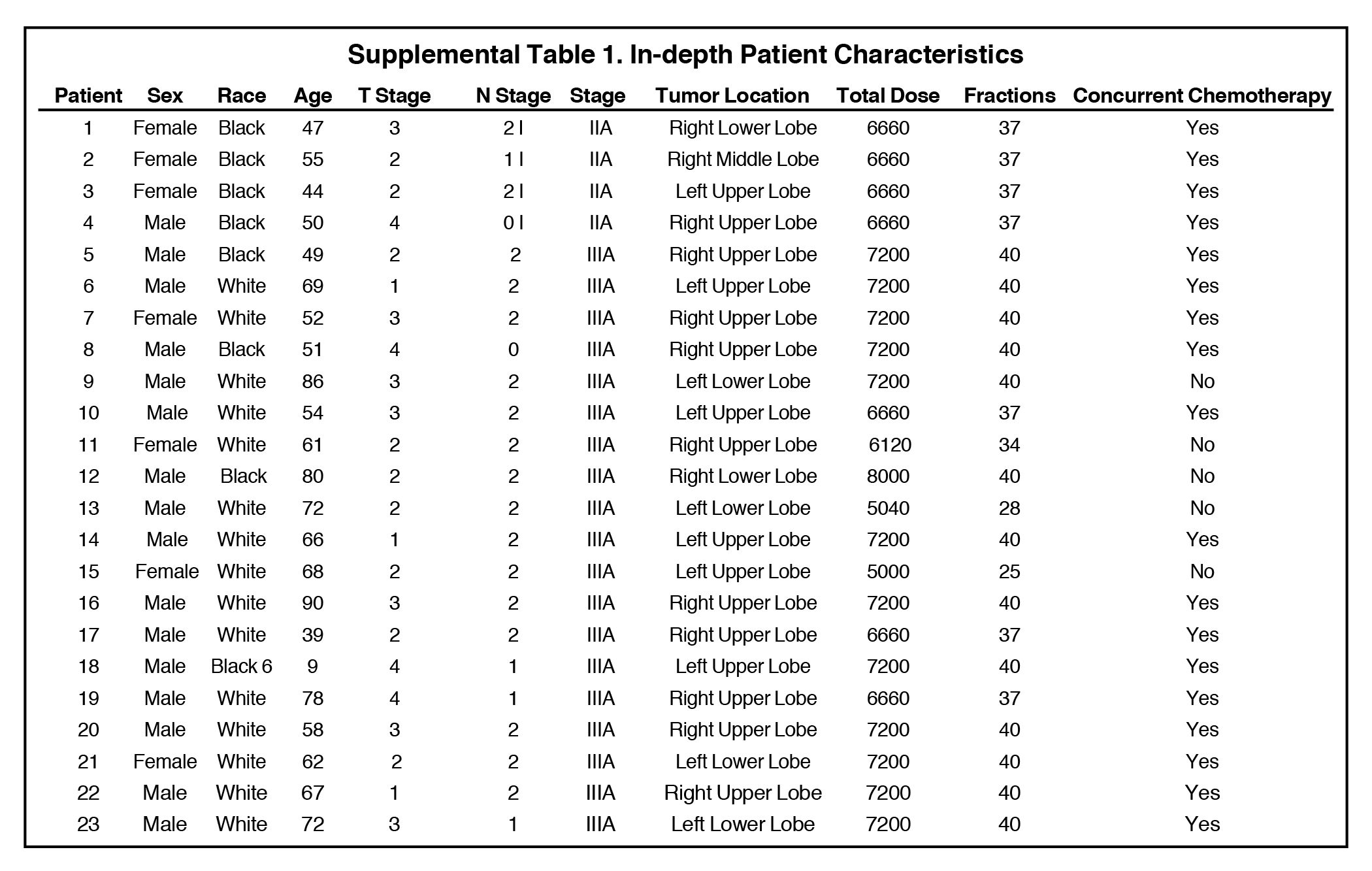
Mean age for the cohort was 62.6 years (range 39-90, median age 62; see Table 1 and Supplemental Table 1). Regarding tumors, 13% were T1, 39% T2, 30% T3, and 17% T4; 9% of tumors were N0, 17% N1, 74% N2. All patients had stage IIIA disease. Median prescribed dose was 72 Gy (mean 68.3 Gy, range 50-80 Gy). Of note, this median dose reflects most patients being treated with institutional prospective dose escalation protocols.
3DCRT and IMRT Plan Comparisons
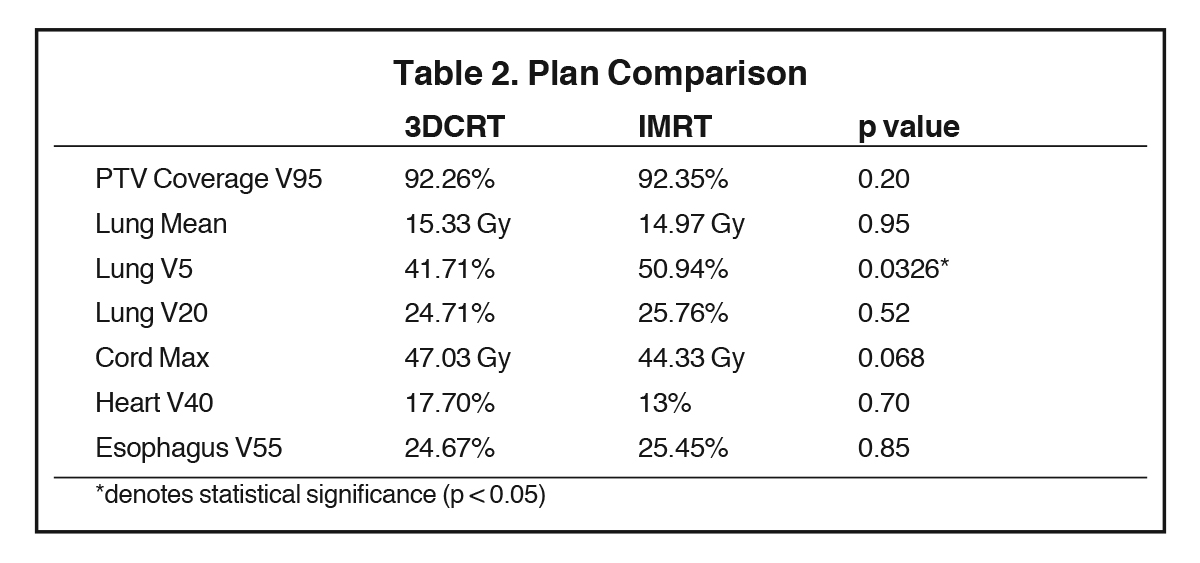
The generated 3DCRT and IMRT plans were compared to ensure that plans were similar in meeting overall dose constraints (Table 2). PTV V95 coverage was similar between 3DCRT (92.3%) and IMRT (92.4%) plans (p = 0.20). Mean lung dose and lung V20 were similar between 3DCRT and IMRT plans: 15.3 vs. 15.0 Gy, p = 0.95; and 24.7% vs. 25.8%, p = 0.52, respectively. IMRT plans delivered a higher lung V5 compared to 3DCRT (50.9% vs. 41.7%, p = 0.03). There were no significant differences in the cord max (47.0 vs. 44.3 Gy, p = 0.07), heart V40 (17.7% vs. 13.0%, p = 0.70), and esophagus V55 (24.7% vs. 25.5%, p = 0.85).
Elective Nodal Station Dosimetric Comparisons
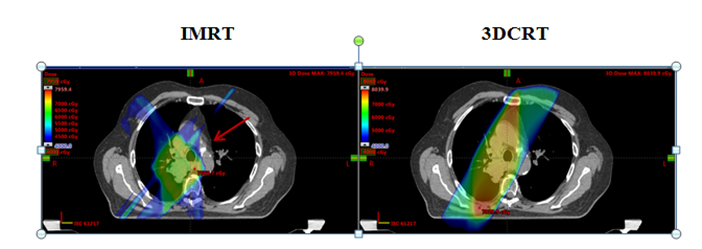
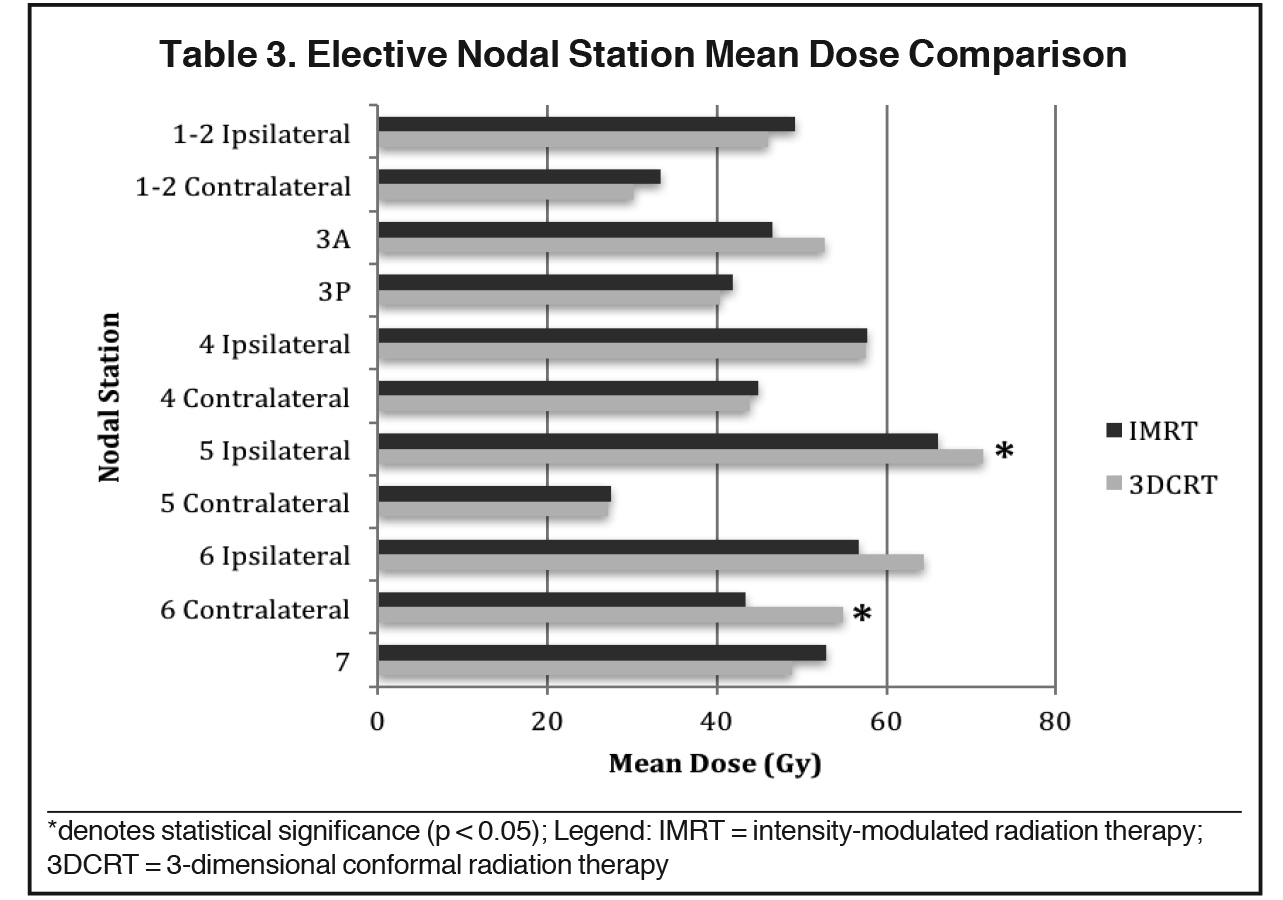
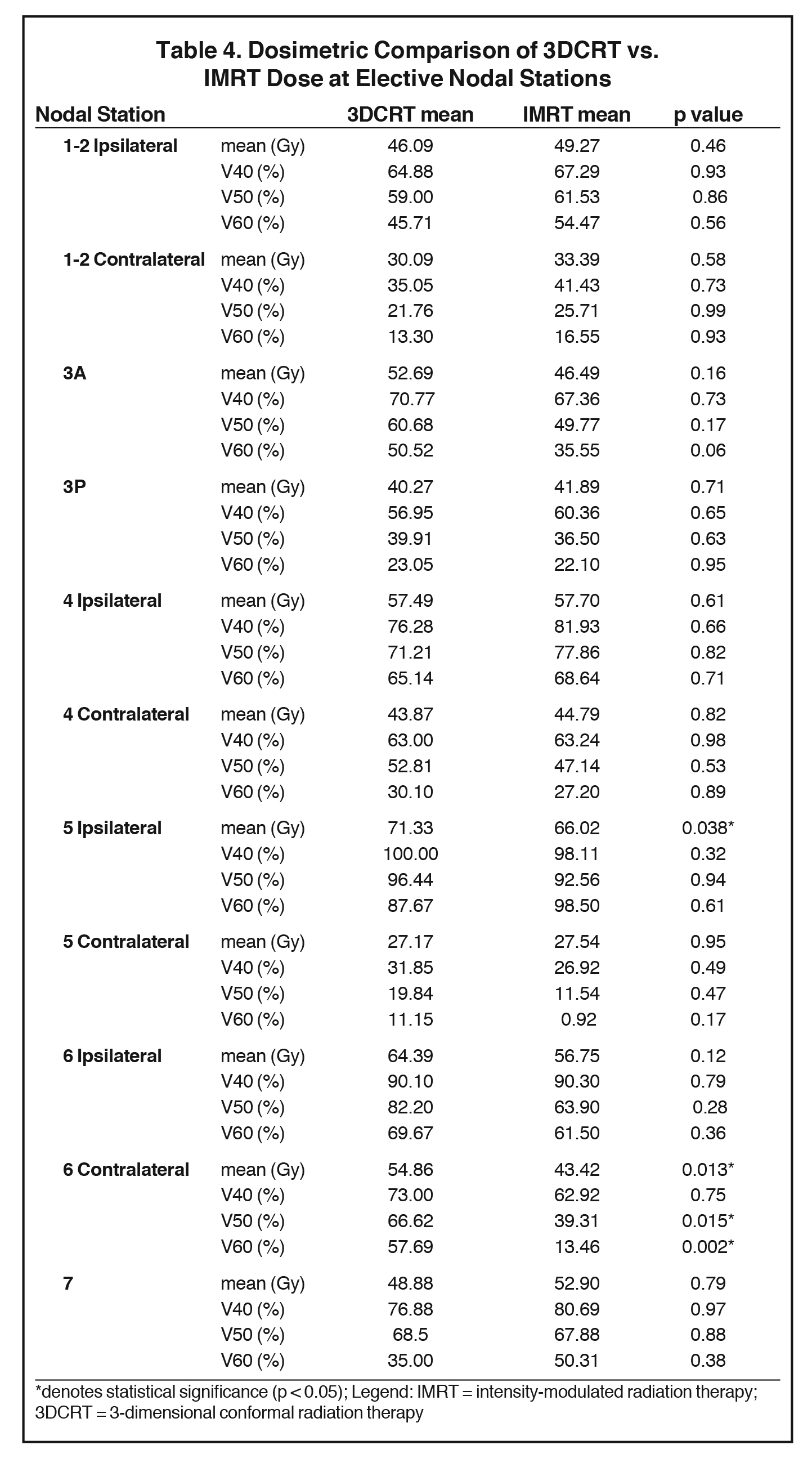
The mean dose at each EN station ranged from 27.2 to 71.3 Gy for 3DCRT and 27.5 to 66 Gy for IMRT (Table 3). No significant differences in V40, V50 or V60 of most EN stations were found between 3DCRT and IMRT plans, except for contralateral station 6 (right-sided tumors) and ipsilateral station 5 nodes (left-sided tumors). For contralateral station 6, V50 and V60 were significantly less with IMRT than 3DCRT plans (V50: 39.3% vs. 66.6%, p = 0.015 and V60: 13.5% vs. 57.7%, p = 0.002). The mean dose of ipsilateral station 5 and contralateral station 6 nodes were also lower with IMRT vs. 3DCRT: 66 vs. 71.3 Gy, p = 0.038 and 43.4 vs, 54.9 Gy, p = 0.013, respectively (Table 4, Figure 1). Aside from these differences in levels 5 and 6 LNs, primary tumor laterality and level/location did not influence incidental irradiation dose to ENs.
Clinical Outcomes
At a median follow-up of 21 months from radiation completion, 8 patients (34.8%) were alive, of whom 5 patients (21.7%) had no evidence of disease. Six patients (26.1%) had locoregional-only recurrences, 5 patients (21.7%) had distant disease at progression, and 3 patients (13.0%) had both locoregional and distant disease. Of the patients with locoregional-only recurrences, only 1 patient (4%) failed in the regional LNs alone without intrathoracic disease progression. Unlike the other patients who were treated definitively, this patient had a stage IIIA (pT2N2M0) right upper lobe adenocarcinoma for which she underwent right upper lobectomy and lymph node dissection demonstrating nodal disease at levels 10R and 4R. Given the pN2 disease, she received adjuvant sequential chemotherapy followed by radiation (total dose 61.2 Gy due to positive margin). The patient subsequently failed in LN regions (supraclavicular, station 5) outside of the initially involved nodal stations. However, of note, the supraclavicular LNs would not have been routinely included in the EN volume for this patient with a stage IIIA right-sided primary tumor.
Discussion
With the demonstration of low EN failure rates when treating locally advanced NSCLC with IFRT using 3DCRT,7-10 many physicians have chosen to deliver IFRT to avoid the higher rates of esophagitis and radiation pneumonitis associated with ENI.6,23 However, while IMRT use is increasingly adopted to treat NSCLC, it remains to be established whether IMRT confers the same or similar benefit of incidental irradiation as 3DCRT. Our dosimetric study demonstrates that IMRT can be safely delivered without significant concern for increased risk of nodal failures since EN irradiation does not appear to be compromised. However, caution should be exercised when delivering IFRT with IMRT if there is high risk for subclinical disease in the level 5 and 6 nodal regions.24
We found that IMRT delivers similar incidental radiation doses as 3DCRT to EN stations 2, 3, 4 and 7. However, IMRT delivered less incidental doses to station 5 and 6 nodes vs. 3DCRT. These differences were particularly profound in the ≥ 50 Gy and ≥ 60 Gy dose regions, where IMRT delivered 41% and 77% less dose, respectively, to level 6 nodes, when compared to 3DCRT. Additionally, the mean dose to contralateral station 6 was more than 10 Gy less with IMRT than 3DCRT. At a median follow-up of 21 months, 6 (26%) of our patients had locoregional disease progression, with only 1 (4%) patient having isolated EN failure. While our study objective and focus are on dosimetric comparisons, our exploration of patterns of regional failure with IMRT in locally advanced NSCLC supports a low rate of EN failures.
The low rates of isolated EN failures when treating NSCLC with IFRT using 3DCRT have largely been attributed to incidental nodal irradiation. Rosenzweig and colleagues studied patients treated with 3DCRT without ENI and demonstrated an EN failure rate of only 6%, with some of those failures occurring in supraclavicular nodes that would not be electively targeted in modern ENI fields.9 In a prospective study by Yuan et al, stage III NSCLC patients had an EN failure rate of 4% at 5 years in their ENI arm vs. 7% in the IFRT arm.7 Kimura and colleagues attributed their EN failure rate of only 8% when treating IFRT with 3DCRT to a median incidental nodal dose ≥ 40 Gy in the majority of EN stations.11 Finally, Kepka et al reported a strong dose-response relationship for EN control, with the majority of failures occurring in nodal regions receiving < 50 Gy.22 Our study of IMRT, rather than 3DCRT patients, confirms similar findings, with comparable incidental radiation doses overall in the IMRT plans, and only a 4% rate of isolated regional nodal failure.
Given the low rates of nodal failure with IFRT, many physicians have adopted this technique to reduce treatment-related toxicities without compromising clinical efficacy. Our institution and others have demonstrated a lower rate of esophageal and pulmonary toxicity when treating with IFRT vs. ENI while retaining similar rates of EN control, primary tumor local control, and overall survival.13,25 To further reduce treatment-associated morbidity, more conformal IFRT delivery with IMRT has been increasingly adopted nationwide.15,17 Randomized data from prospective trials also support decreased toxicity when treating NSCLC with IMRT. In a secondary analysis of RTOG 0617, patients treated with IMRT vs. 3DCRT were found to have less decline in quality of life (21% vs 45%, p = 0.003).18,26
However, while the adoption of IMRT is increasing,15,17 the established literature that assesses the impact of IFRT on mediastinal nodal failures has largely emerged during the pre-IMRT era. Fleckenstein et al conducted a dosimetric analysis of incidental nodal irradiation in stage II-III NSCLC patients and reported a lower total dose to adjacent EN stations with IMRT compared to 3DCRT plans (40 vs. 44 Gy, respectively).27 However in this study, all nonelective LNs were grouped into 1 composite volume, and dose to each station was not compared separately. In our study, we further this dosimetric analysis by assessing a novel comparison of individual EN stations allowing us to better understand which stations are at highest risk for failure when treating with IMRT. Additionally, Martinussen and colleagues reported a low rate of isolated nodal failures (2.2%) in stage III NSCLC patients treated IMRT, though this study did not quantify the incidental radiation dose to EN stations.28
To our knowledge, this is the first study that assesses the patterns of nodal failure in locally advanced NSCLC treated with IMRT in the context of dosimetric differences to individual EN stations between 3DCRT vs. IMRT plans for each patient. Therefore, our dosimetric comparison of incidental nodal irradiation is particularly relevant in clinical considerations for the treatment of NSCLC patients with more conformal IMRT. Our study also highlights the importance of mediastinal lymph node staging to appropriately treat all gross nodal disease when ENI is omitted.
A few limitations of our analysis should be noted. First, the retrospective nature of the study leaves it susceptible to selection bias. However, the comparison between 3DCRT and IMRT plans on the same patient allowed all patients to serve as their own internal control. Second, our overall sample size was small due to our strict inclusion and exclusion criteria. However, these narrow criteria allowed us to examine a relatively homogenous cohort, allowing for fewer patients to be needed for a meaningful dosimetric comparison. Furthermore, we excluded stage IIIB patients with contralateral nodal disease because even fewer differences between incidental nodal irradiation would have been seen between IMRT and 3DCRT if these patients were included in this analysis. Given the small sample size, a detailed analysis on variables associated with differences in incidental dose was largely limited. Third, given the difference in techniques, the present analysis cannot be extrapolated to the use of volumetric modulated arc therapy (VMAT) or proton therapy, which is also increasingly being used in locally advanced NSCLC29,30 but which is also surrounded by a theoretical risk of EN failures. Finally, our patients were treated prior to the publication of RTOG 061718 and predominantly on institutional dose escalation protocols resulting in potentially higher prescription doses than may be used today. However, escalated doses are still common today31 with trials such as RTOG 1308 allowing prescription doses up to 70 Gy.19
In conclusion, our dosimetric analysis demonstrates that IFRT using IMRT offers comparable microscopic incidental irradiation doses as 3DCRT to EN regions. These data are encouraging for the continued use of IFRT with IMRT in NSCLC when clinically indicated, and support the promising advantage of IMRT in conformal dose escalation while limiting treatment-related toxicities. However, when treating patients with a high risk of subclinical disease in levels 5 and 6, such as patients with left upper lobe and left central tumors,24,32,33 IMRT should be used cautiously given the reduced incidental dose to these stations seen in this study with IMRT compared to 3DCRT. While only 1 patient in our study had an isolated nodal failure, given our small sample size future studies should evaluate the clinical correlations of these dosimetric findings to assess EN control after treatment with involved field IMRT.
References
- Curran WJ, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452-1460. doi:10.1093/jnci/djr325.
- National Comprehensive Cancer Network (NCCN) Guidelines: Non-Small Cell Lung Cancer (Version 4.2016). doi:http://dx.doi.org/10.1016/0011-5029(88)90024-7.
- Halperin EC, Brady LW, Perez CA, Wazer DE. Perez & Brady’s Principles and Practice of Radiation Oncology. Philadelphia, PA: Wolters Kluwer Health; 2013. http://books.google.com/books?id=GEwlAgAAQBAJ. Accessed July 2016.
- Marks LB, Prosnitz LR. Assessing the impact of elective regional radiotherapy on survival. Cancer J Sci Am. 1999;5(2):92-100.
- De Ruysscher D, Faivre-finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer Recommendations for Planning and Delivery of High-Dose, High-Precision Radiotherapy for Lung Cancer. J Clin Oncol. 2016;28(36). doi:10.1200/JCO.2010.30.3271.
- Grills IS, Yan D, Martinez A, et al. Potential for reduced toxicity and dose escalation in the treatment of inoperable non–small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol. 2003;57(3):875-890. doi:10.1016/S0360-3016(03)00743-0.
- Yuan S, Sun X, Li M, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol. 2007;30(3):239-244. doi:10.1097/01.
- Chen M, Hayman J, Ten Haken RK, et al. Long-term results of high-dose conformal radiotherapy for patients with medically inoperable T1-3N0 non-small-cell lung cancer: is low incidence of regional failure due to incidental nodal irradiation? Int J Radiat Oncol Biol Phys. 2006;64(1):120-126. doi:10.1016/j.ijrobp.2005.06.029.
- Rosenzweig KE, Sura S, Jackson A, Yorke E. Involved-field radiation therapy for inoperable non small-cell lung cancer. J Clin Oncol. 2007;25(35):5557-5561. doi:10.1200/JCO.2007.13.2191.
- Rosenzweig KE, Sim SE, Mychalczak B, et al. Elective nodal irradiation in the treatment of non-small-cell lung cancer with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50(3):681-685. http://www.ncbi.nlm.nih.gov/pubmed/11395236.
- Kimura T, Togami T, Nishiyama Y, et al. Impact of incidental irradiation on clinically uninvolved nodal regions in patients with advanced non-small-cell lung cancer treated with involved-field radiation therapy: does incidental irradiation contribute to the low incidence of elective nodal failu. Int J Radiat Oncol Biol Phys. 2010;77(2):337-343. doi:10.1016/j.ijrobp.2009.05.039.
- Zhao L, Chen M, Ten Haken R, et al. Three-dimensional conformal radiation may deliver considerable dose of incidental nodal irradiation in patients with early stage node-negative non-small cell lung cancer when the tumor is large and centrally located. Radiother Oncol. 2007;82(2):153-159. doi:10.1016/j.radonc.2007.01.006.
- Jiang Z-Q, Yang K, Komaki R, et al. Long-term clinical outcome of intensity-modulated radiotherapy for inoperable non-small cell lung cancer: the MD Anderson experience. Int J Radiat Oncol Biol Phys. 2012;83(1):332-339. doi:10.1016/j.ijrobp.2011.06.1963.
- Govaert S LA, Troost EG, Schuurbiers OC, et al. Treatment outcome and toxicity of intensity-modulated (chemo) radiotherapy in stage III non-small cell lung cancer patients. Radiat Oncol. 2012;7:150. doi:10.1186/1748-717X-7-150.
- Shirvani SM, Jiang J, Gomez DR, et al. Intensity modulated radiotherapy for stage III non-small cell lung cancer in the United States: predictors of use and association with toxicities. Lung Cancer. 2013;82(2):252-259. doi:10.1016/j.lungcan.2013.08.015.
- Chang JY. Intesity-modulated radiotherapy, not 3 dimensional conformal, is the preferred technique fort treating locally advanced lung cancer. Semin Radiat Oncol. 2015;25(2):110-116. doi:10.1016/j.semradonc.2014.11.002.
- Harris JP, Murphy JD, Hanlon AL, et al. A population-based comparative effectiveness study of radiation therapy techniques in stage III non-small cell lung cancer. Radiat Oncol Biol. 2014;88(4):872-884. doi:10.1016/j.ijrobp.2013.12.010.
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial p. Lancet Oncol. 2015;16(2):187-199. doi:10.1016/S1470-2045(14)71207-0.
- Giaddui T, Chen W, Yu J, et al. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol. 2016;11(1):66. doi:10.1186/s13014-016-0640-8.
- Simone CB 2nd, Houshmand S, Kalbasi A, et al. PET-based thoracic radiation oncology. PET Clin. 2016;11(3):319-332. doi:10.1016/j.cpet.2016.03.001.
- Chapet O, Kong F-M, Quint LE, et al. CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys. 2005;63(1):170-178. doi:10.1016/j.ijrobp.2004.12.060.
- Kepka L, Maciejewski B, Withers RH. Does incidental irradiation with doses below 50 gy effectively reduce isolated nodal failures in non-small-cell lung cancer: dose-response relationship. Int J Radiat Oncol Biol Phys. 2009;73(5):1391-1396. doi:10.1016/j.ijrobp.2008.07.070.
- Shirvani SM, Juloori A, Allen PK, et al. Comparison of 2 common radiation therapy techniques for definitive treatment of small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87(1):139-147. doi:10.1016/j.ijrobp.2013.05.040.
- Billiet C, De Ruysscher D, Peeters S, et al. Patterns of locoregional relapses in patients with contemporarily staged stage III-N2 NSCLC treated with induction chemotherapy and resection: implications for postoperative radiotherapy target volumes. J Thorac Oncol. June 2016. doi:10.1016/j.jtho.2016.05.037.
- Fernandes AT, Shen J, Finlay J, et al. Elective nodal irradiation (ENI) vs. involved field radiotherapy (IFRT) for locally advanced non-small cell lung cancer (NSCLC): a comparative analysis of toxicities and clinical outcomes. Radiother Oncol. 2010;95(2):178-184. doi:10.1016/j.radonc.2010.02.007.
- Movsas B, Hu C, Sloan J, et al. Quality of life analysis of a radiation dose-escalation study of patients with non-small-cell lung cancer: A secondary analysis of the Radiation Therapy Oncology Group 0617 randomized clinical trial. JAMA Oncol. 2015;48202:1-9. doi:10.1001/jamaoncol.2015.3969.
- Fleckenstein J, Eschler A, Kremp K, et al. Dose distribution and tumor control probability in out-of-field lymph node stations in intensity modulated radiotherapy ( IMRT ) vs 3D-conformal radiotherapy (3D-CRT of non-small-cell lung cancer :an in silico analysis. Radiat Oncol. 2015:1-7. doi:10.1186/s13014-015-0485-6.
- Martinussen HMA, Reymen B, Wanders R, et al. Is selective nodal irradiation in non-small cell lung cancer still safe when using IMRT? Results of a prospective cohort study. Radiother Oncol. 2016;121(2):322-327. doi:10.1016/j.radonc.2016.10.001.
- Simone CB 2nd, Rengan R. The use of proton therapy in the treatment of lung cancers. Cancer J. 2014;20(6):427-432. doi:10.1097/PPO.0000000000000080.
- Chang JY, Jabbour SK, De Ruysscher D, et al. Consensus Statement on Proton Therapy in Early-Stage and Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):505-516. doi:10.1016/j.ijrobp.2016.01.036.
- Hall MD, Gabriel PE, Guo W, et al. Changing patterns of care for locally advanced non-small cell lung cancer (NSCLC): implications for quality initiatives. J Clin Oncol (Meeting Abstr. 2016;34(7_suppl):302. http://meeting.ascopubs.org/cgi/content/abstract/34/7_suppl/302.
- Kotoulas CS, Foroulis CN, Kostikas K, et al. Involvement of lymphatic metastatic spread in non-small cell lung cancer accordingly to the primary cancer location. Lung Cancer. 2004;44(2):183-191. doi:10.1016/j.lungcan.2003.10.012.
- Watanabe Y, Shimizu J, Tsubota M, Iwa T. Mediastinal spread of metastatic lymph nodes in bronchogenic carcinoma. Mediastinal nodal metastases in lung cancer. Chest. 1990;97(5):1059-1065.
Citation
S S, JT W, W Z, AF S, EP X, JP C, S B, R R, II SC, S A. Incidental nodal irradiation in locally advanced non-small cell lung cancer treated with involved-field IMRT. Appl Radiat Oncol. 2017;(4):21-27.
December 14, 2017