Improving the therapeutic index for nonoperable esophageal cancer patients with modern radiation technologies
Images
SA-CME credits are available for this article here.
Esophageal cancer (EC) is one of the leading causes of cancer-related death worldwide.1 Approximately 50% of newly diagnosed EC patients are not surgical candidates due to extensive locoregional disease, distant metastasis, and/or being medically unfit. Definitive chemoradiation (CRT) became a standard of care many years ago for nonsurgical patients based on results of the Radiation Therapy Oncology Group (RTOG) 85-01 randomized trial that demonstrated superior overall survival (OS) with 50 Gy plus 4 cycles of 5-fluorouracil (5-FU) and cisplatin compared with 64 Gy alone; 5-year survival was 26% vs. 0%, respectively.2 There is also an apparent benefit of concurrent chemotherapy in elderly EC patients.3,4 The standard radiation dose in nonoperable EC patients has not changed for decades ever since the Intergroup (INT) 0123 trial reported no survival benefit in escalating dose from 50.4 Gy in 28 fractions to 64.8 Gy in 36 fractions, both given with 4 cycles of 5-FU and cisplatin.5
It is important to recognize that these seminal trials were conducted many years ago using 2-dimensional (2D) x-ray radiation therapy (RT) prior to dramatic improvements in technology. Whereas generous treatment ports were used in the 2D treatment era, the development of 3-dimensional conformal radiation therapy (3DCRT) and intensity-modulated radiation therapy (IMRT) has enabled highly conformal treatment delivery and the lowering of normal tissue dose.6-8 As opposed to x-rays, which exponentially deposit dose in tissue along the beam path resulting in exit dose in surrounding normal tissues (eg, heart and lungs), protons deposit more efficiently as they lose the majority of their energy near the end of their beam range as they come to rest. This results in a sharp rise in absorbed dose called the “Bragg peak” followed by a sharp dose falloff. Proton beam therapy (PBT) represents another step in the evolutionary ladder of radiation technology.9 Lastly, present-day treatment planning techniques including heterogeneity corrections, high-quality image guidance including cone-beam computed tomography (CT), and the use of tighter margins have also contributed to reducing dose outside of the target volume.10,11
Herein we review how contemporary radiation technologies provide opportunities for potential improvements in the therapeutic index, including both reduced toxicity and higher tumor control, for nonoperable EC patients receiving definitive CRT.
Reducing Cardiopulmonary Toxicity
Delivering RT to the esophagus is challenging due to its central location within the chest, surrounded by multiple critical structures, notably the lungs and heart. There is heightened awareness, particularly from outcomes of breast and lung cancer patients, that increasing heart and lung dose, even in the low dose range, can significantly increase the risk of cardiopulmonary toxicity (CPT).12-14 As such, efforts have focused on evaluating whether modern radiation technologies can spare both of these critical organs, and whether any dosimetric differences are clinically meaningful.
Intensity-Modulated Radiation Therapy
IMRT delivers improved conformality and reduced normal organ dose compared to less sophisticated techniques for EC patients as demonstrated by multiple treatment planning studies.15,16 A recently published analysis of 7 dosimetric studies demonstrated dramatic lung and heart sparing with IMRT vs. 3DCRT; for example, IMRT resulted in significantly lower average irradiated volume of the heart among patients treated to at least 50 Gy (mean difference: 4.78 cc [95% CI: 0.88-8.68], P = .02).17
The ability of IMRT to minimize dose outside of the target volume appears to be clinically meaningful. A study published from the phase II/III SCOPE1 (Study of Chemoradiotherapy in OesoPhageal cancer with Erbitux) trial found that higher OS was strongly associated with a higher conformality index and that plan quality was strongly related to receiving IMRT (vs. 3DCRT).18 Freilich et al reported reduced grade 3 or higher toxicity (OR 0.51; P = 0.05), defined as any hospitalization, feeding tube, or > 20% weight loss, with IMRT vs. 3DCRT.19 An analysis of 676 patients treated at MD Anderson Cancer Center (MDACC) reported significantly improved OS (HR 0.72, p < .001) with IMRT compared with 3DCRT. Although there was no difference in cancer-related or pulmonary-related death, patients receiving 3DCRT had a significantly greater risk of cardiac death (5-year estimate, 11.7% (3DCRT) vs. 5.4% (IMRT), Gray’s test, P = 0.0029).20 Lastly, an analysis of two large cancer center registries including over 2500 elderly patients further supports the advantage of IMRT; on propensity score inverse probability of treatment weighting multivariate analysis, IMRT was associated with less all-cause, other-cause, and cardiovascular mortality compared to 3DCRT.21
Despite these retrospective data suggesting a large and significant benefit of IMRT, a small randomized trial from China of 60 patients reported significant improvements in complete response rate and reduction in lung V20 and V30 in patients receiving IMRT, but did not report improvements in OS.22 However, comprehensive evaluation of cardiac-related mortality was not performed.
Collectively, these largely retrospective data suggest that IMRT should be considered over 3DCRT because of reduced CPT and potentially improved OS. There is a need to confirm these benefits in a prospective manner.
Proton Beam Therapy
he published literature has demonstrated benefits of PBT compared to x-ray therapy in sparing critical thoracic organs. Zhang et al compared passive scattering PBT with fixed-field IMRT plans prescribed to 50.4 Gy for 15 distal esophageal cancer patients.23 Compared to IMRT plans, PBT plans had improved lung sparing at low-to-moderate doses from V5-V20, as well as mean lung dose. Lung sparing was the greatest at the lowest dose levels; PBT reduced V5 lung dose relatively by 36% to 70% depending on the beam arrangements. Heart V40 was more modestly reduced (up to 22% relatively) with PBT. Shiraishi et al published a detailed analysis of dose delivered to cardiac substructures in EC patients, concluding that PBT could deliver markedly reduced dose to many, but not all, of these substructures compared to x-ray techniques.24
PBT delivered with pencil-beam scanning (PBS) offers increased dose conformality compared to passive scattering technique. A study from MDACC demonstrated significant lung and heart sparing in the low-to-moderate range with various PBS-PBT beam arrangements compared to IMRT.25 PBS-PBT delivered with a single posterior field (SPF) with volumetric rescanning has been proposed to minimize normal organ dose.26 Zeng et al from University of Washington demonstrated that when compared to anterior-posterior/posterio-anterior (AP/PA) beams, the SPF approach significantly spared more heart by approximately 50%, and when compared to PA/left posterior oblique (PA/LPO) beams, the SPF approach significantly spared more lungs by approximately 40%.
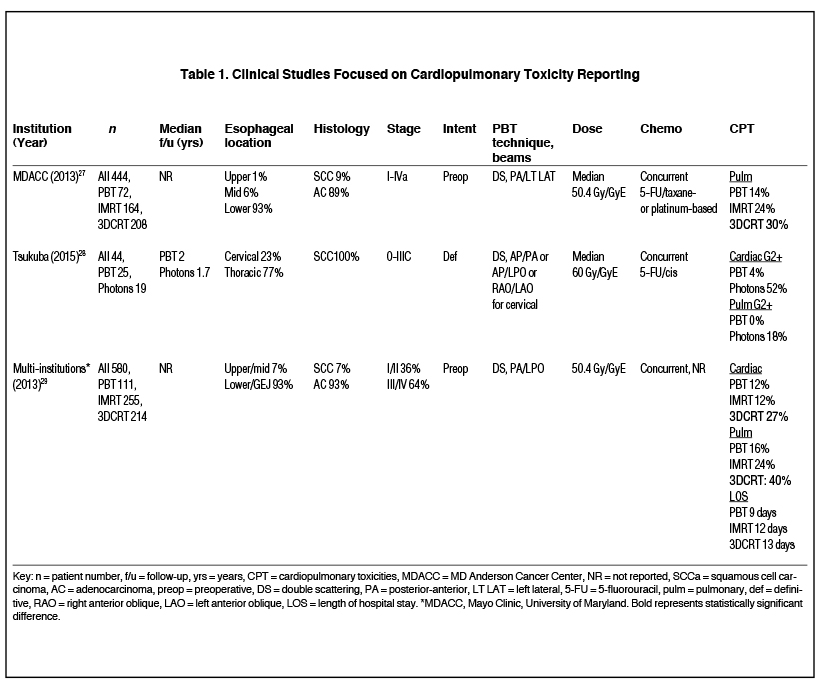
Although dosimetric superiority does not always translate into clinically significant differences, the published literature demonstrates reductions in CPT with PBT (Table 1). Wang et al reviewed 444 patients treated with preoperative PBT (n = 72), IMRT (n = 164), and 3DCRT (n = 208) with concurrent chemotherapy.27 Pre-treatment lung capacity and radiation modality were found to be independent predictors of pulmonary complications. PBT-treated patients had the lowest rate of postoperative pulmonary complications (14%) compared to those who received IMRT (24%) or 3DCRT (30%). However, only the PBT and 3DCRT differences were statistically significantly different, leaving up for debate whether there are meaningful differences between PBT and IMRT. The authors concluded that the lung sparing from PBT was likely responsible for the decrease in pulmonary complications since mean lung dose was found to correlate with pulmonary complications. No differences in cardiac complications were observed. Investigators from the University of Tsukuba also reported reduced pulmonary toxicity among PBT vs. x-ray patients, although they found reduced cardiac toxicity with PBT, in contrast to the MDACC study.28
To further evaluate their findings of decreased CPT with PBT, MDACC pooled their data with two other academic institutions and analyzed a total of 580 lower esophageal/GEJ cancer patients (111 PBT, 255 IMRT, 214 3DCRT).29 The type of radiation modality was associated with CPT on multivariate analysis. Specifically, PBT patients had significantly less pulmonary toxicity compared with 3DCRT patients (16% vs. 40%) although there was no statistically significant difference compared to IMRT patients (24%). As opposed to the initial MDACC study, this pooled analysis reported fewer cardiac complications in PBT patients when compared to 3DCRT patients (12% vs. 27%), although there was no difference when compared to IMRT patients (12%).
Reducing Hematologic Toxicity
There is increasing interest in studying the effects of radiation modality on hematologic toxicity (HT). While most of the body’s bone marrow (BM) is in the pelvis, approximately 35% of the active BM resides in the thoracic vertebrae (TV).30 The risk of developing ≥ grade 2 HT such as leukopenia and neutropenia has been associated with BM irradiation in both pelvic and thoracic RT patients.31-34
In a dosimetric planning study, IMRT and PBT were recently reported by a group from the United Kingdom as superior to 3DCRT in overall BM sparing.35 PBT, however, was the only modality to provide significant sparing in the very loswest dose range (ie, bone V10). Warren et al performed a study including 12 patients with mid-esophageal tumors and compared the BM (TV, sternum, scapulae, ribs, clavicles) and TV (T1-T12) doses among 3DCRT, volumetric-modulated arc therapy (VMAT) IMRT, simultaneous integrated boost (SIB)-VMAT, PBS-PBT, and TV-sparing (TVS) VMAT plans.35 Only the PBS plan showed clinically significant sparing of the bone V10, V20 and mean dose compared to all techniques. However, the PBS plans showed no dosimetric advantage over the TVS-VMAT plans for any TV dose-volume metrics. While the clinical relevance of these results remains unclear, this study provides evidence that PBT can substantially reduce HT, depending on the bone OAR being spared.
Radiation-induced adverse effects on the immune system include severe lymphopenia and impaired recruitment of tumor-infiltrating lymphocytes (TILs), which have been correlated with unfavorable clinical outcomes.36-39 Because lymphocytes are exquisitely radiosensitive to low dose (ie, V5-V15), a priority should be to minimize radiation exposure especially to large volumes of the blood and, therefore, lymphocytes that circulate through the heart and lungs at any given time.40,41 The importance of this was supported by a retrospective analysis of 711 non-small cell lung cancer patients who received definitive RT and found an association between lung V5, lymphocyte nadir, and survival.42 Shiraishi et al compared the risk of radiation-induced grade 4 lymphopenia between PBT and IMRT patients with EC (n = 136 in each group) using propensity matching based on key clinical characteristics.40 PBT patients had markedly less frequent grade 4 lymphopenia compared to IMRT patients (17.6% vs. 40.4%; p < 0.0001). On multivariate analysis, PBT was found to be an independent predictor for grade 4 lymphopenia (OR 0.29; 95% confidence interval, 0.16 to 0.52; p < 0.0001). However, grade 4 lymphopenia was not found to be an independent predictor for poorer OS.
Dose Escalation
Rationale for Dose Escalation
Local control (LC) is poor for EC patients treated with definitive CRT.2,5 Adenocarcinomas and squamous cell carcinomas both recur in the original gross tumor volume in about 40% of patients.43 Radiation dose escalation for such patients remains controversial based on the results of the aforementioned INT trial in which patients in the high dose arm had worse OS.5 However, 7 of the 11 deaths during RT occurred prior to delivery of 50.4 Gy, making it impossible for dose escalation to be responsible for the higher mortality rate. Also, with longer follow-up, there was a significantly higher number of deaths not attributable to EC in the high dose arm compared with the standard dose arm (13 vs. 3; P < 0.01). Hence, the results of this trial cannot be used to conclude that radiation dose escalation does not offer clinical benefit, largely because of the technological limitations of the era in which it was conducted. For now, we can only speculate whether the results of this trial would have differed if modern techniques were used.44
The era of 3D planning has seen increasing interest in exploring whether dose escalation specifically to gross disease offers therapeutic benefit in nonoperable EC patients. This strategy is based on studies showing that at least 75% of local recurrences after definitive CRT prescribed to 50.4 Gy occur within the gross tumor volume (GTV) and not within electively treated regions, suggesting that selective delivery of higher dose to gross disease may improve outcomes.45,46
Intensity-Modulated Radiation Therapy
IMRT can selectively increase dose to the GTV while reducing dose to normal organs.6,47A potential benefit of IMRT delivered with SIB is that fraction sizes > 2 Gy prescribed to the GTV may have a radiobiological advantage in counteracting accelerated repopulation and more effectively eliminating cancer stem cells.48 Early results from a Chinese phase 2 trial that prescribed concurrent chemotherapy plus 63 Gy to the GTV and 50.4 Gy to the PTV, all in 28 fractions using IMRT-SIB, were encouraging; locoregional control at 3 years was 67.5% and no grade 4-5 toxicity occurred.49 Investigators from MDACC subsequently published outcomes of a phase 1/2 trial that employed IMRT-SIB over 28 fractions with 63 Gy being the maximum tolerated dose.50 After a median follow-up of 13.3 months, 11 (29%) patients experienced local recurrence and the rate of acute esophagitis was similar to historical control. When compared to 97 similar nonoperable EC patients who received a total of 50.4 Gy, there was significantly improved LC in patients who received a boost. This trial included patients mostly treated with IMRT, but a minority received PBT.
Several ongoing trials are evaluating the role of dose escalation based on tumor response to initial therapy as determined by PET/CT. A phase 1 trial from China (NCT03113214) is evaluating PET/CT-directed hyperfractionated radiation dose escalation and concurrent carboplatin/paclitaxel with total doses ranging from 57.2 to 93.2 Gy prescribed to residual tumor after an initial 50 Gy. The SCOPE2 phase 2/3 trial (NCT02741856) uses PET/CT response after initial cisplatin/capecitabine to 50 Gy in 25 fractions vs 60 Gy in 25 fractions.
Proton Beam Therapy
The University of Tsukuba was the first institution to publish clinical outcomes using PBT for esophageal cancer in 1994, which was given in a dose-escalated fashion.51 Koyama et al treated 15 patients with superficial and advanced esophageal cancer (93% SCC) using definitive hypofractionated passive scattering PBT alone to 80.4 gray equivalent (GyE) with a single AP field either as a boost after 3DCRT or as a single full PBT course. OS at 5 years was 27% with 67% LC for advanced tumors. Over the next several years the same institution updated their clinical experience with hypofractionated passive scattering PBT in a series of publications.52-54 A hypo- fractionated regimen and single AP or AP/PA beam approach were employed primarily due to resource allocation and technology limitations. For locally advanced tumors, 5-year LC was 29% to 43% and 5-year OS was 13% to21% in these series.
There is continued interest in esophageal dose escalation with PBT. Two ongoing trials from University of Florida and University of Pennsylvania are investigating the potential toxicity reduction and safety of PBT escalation in both unresectable and resectable esophageal cancer. A phase 2 trial from University of Florida (NCT03234842) is treating patients to 59.4 GyE with concurrent carboplatin/paclitaxel and PBT. Patients who decline or are not able to receive PBT will be treated on a comparator x-ray cohort. The primary endpoint of this study is to assess the differences in lung function as defined by reduction in diffusing capacity of the lung for carbon monoxide (DLCO) between PBT and x-rays. A phase 1 study from University of Pennsylvania (NCT02213497) is systematically investigating the safety of simultaneous integrated boost dose escalation with PBT in the preoperative setting with 5 dose levels starting at 53.75 GyE and escalating to 62.50 GyE in 25 fractions. Dose-limiting toxicity occurring prior to surgery will be the primary endpoint to inform on a recommended phase 2 dose.
Brachytherapy
Dose escalation using intraluminal brachytherapy as a boost in EC patients treated with curative intent is not commonly used, although it may benefit select patients. A phase 2 RTOG trial of 49 EC patients, nearly all with SCC who received chemoradiation to 50 Gy followed by a brachytherapy boost, demonstrated no difference in survival or local control compared to the historical control.55 Furthermore, a high incidence of life-threatening toxicity (24%) or treatment-related death (10%) occurred. A Japanese randomized trial that included patients with SCC of the esophagus who after 60 Gy received a boost with external beam vs. brachytherapy demonstrated no difference in overall survival.56 However, those with tumors < 5 cm in length had more than twice the cancer-specific survival (64 vs. 31.5%; p = 0.025).
In conclusion, dose escalation using a brachytherapy boost should not be recommended for all EC patients, although it could be reasonable for a subset with limited disease, as endorsed by published guidelines from the American Brachytherapy Society.57
Patient Selection for Dose Escalation
These data suggest that radiation dose escalation may be effective using both x-rays and protons, although all patients may not benefit from higher doses. Variable responses to definitive CRT are well documented, with some patients achieving a complete response while others have persistent disease after 50 to 50.4 Gy. For instance, Ishikawa and colleagues observed more local recurrences (38%) in patients with residual disease seen on endoscopy after 50 GyE who were then dose escalated to 64 to 70 GyE compared with those with no residual disease who were prescribed 60 GyE (5%).58 We are gaining a better understanding of treatment response predictors that include, but are not limited to, tumor stage,59 imaging parameters,60 and intrinsic tumor radiosensitivity,61 although robust clinical decision-making tools are lacking to identify patients for whom radiation dose escalation could be considered. This is clearly an area in need of further study.
Conclusion
Although there is general awareness that modern radiation technologies reduce normal organ dose while permitting safe dose escalation in nonoperable EC patients, consensus is lacking about how these technologies should be routinely employed in the clinic. Well-designed clinical trials are clearly needed to guide clinical decision making in this regard, several of which are being planned (NCT01102088) or underway (NCT01512589).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA.1999;281(17):1623-1627.
- hao L, Zhou Y, Pan H, et al. Radiotherapy Alone or concurrent chemoradiation for esophageal squamous cell carcinoma in elderly patients. J Cancer. 2017;8(16):3242-3250.
- Xu HY, Du Z, Zhou L, et al. Safety and efficacy of radiation and chemoradiation in patients over 70 years old with inoperable esophageal squamous cell carcinoma. Oncol Lett. 2014;7(1):260-266.
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167-1174.
- Welsh J, Palmer MB, Ajani JA, et al. Esophageal cancer dose escalation using a simultaneous integrated boost technique. Int J Radiat Oncol Biol Phy.s 2012;82(1):468-474.
- Chen YJ, Liu A, Han C, et al. Helical tomotherapy for radiotherapy in esophageal cancer: a preferred plan with better conformal target coverage and more homogeneous dose distribution. Med Dosim. 2007;32(3):166-171.
- Deng JY, Wang C, Shi XH, et al. Reduced toxicity with three-dimensional conformal radiotherapy or intensity-modulated radiotherapy compared with conventional two-dimensional radiotherapy for esophageal squamous cell carcinoma: a secondary analysis of data from four prospective clinical trials. Dis Esophagus. 2016;29(8):1121-1127.
- Chuong MD, Hallemeier CL, Jabbour SK, et al. Improving outcomes for esophageal cancer using proton beam therapy. Int J Radiat Oncol Biol Phys. 2016;95(1):488-497.
- Chandra A, Liu H, Tucker, SL, et al. IMRT reduces lung irradiation in distal esophageal cancer over 3D CRT. Int J Radiat Oncol Biol Phys. 200357(2):S384-S385.
- Xiao Y, Papiez L, Paulus R, et al. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73(4):1235-1242.
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
- Taylor CW, Povall JM, McGale P, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys. 2008;72(2):501-507.
- Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387-1394.
- Wang D, Yang Y, Zhu J, et al. 3D-conformal RT, fixed-field IMRT and RapidArc, which one is better for esophageal carcinoma treated with elective nodal irradiation. Technol Cancer Res Treat. 2011;10(5):487-494.
- Nicolini G, Ghosh-Laskar S, Shrivastava SK, et al. Volumetric modulation arc radiotherapy with flattening filter-free beams compared with static gantry IMRT and 3D conformal radiotherapy for advanced esophageal cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2012;84(2):553-560.
- Xu D, Li G, Li H, Jia F. Comparison of IMRT versus 3D-CRT in the treatment of esophagus cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2017. 96(31):e7685.
- Carrington R, Spezi E, Gwynne S, et al. The influence of dose distribution on treatment outcome in the SCOPE 1 oesophageal cancer trial. Radiat Oncol. 2016;11:19.
- Freilich J, Hoffe SE, Almhanna K, et al. Comparative outcomes for three-dimensional conformal versus intensity-modulated radiation therapy for esophageal cancer. Dis Esophagus. 2015;28(4):352-357.
- Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84(5):1078-1085.
- Lin SH, Zhang N, Godby J, et al. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer. 2016;122(6):917-928.
- Lin XD, Shi XY, Zhou TC, Zhang WJ. Intensity-modulated or 3-D conformal radiotherapy combined with chemotherapy with docetaxel and cisplatin for locally advanced esophageal carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(7):1264-1267.
- Zhang X, Zhao KL, Guerrero TM, et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2008;72(1):278-287.
- Shiraishi Y, Xu C, Yang J, Komaki, Lin SH. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother Oncol. 2017;125(1):48-54.
- Welsh J, Gomez D, Palmer MB, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: a dosimetric study. Int J Radiat Oncol Biol Phys. 2011;81(5):1336-1342.
- Zeng YC, Vyas S, Dang Q, et al. Proton therapy posterior beam approach with pencil beam scanning for esophageal cancer: clinical outcome, dosimetry, and feasibility. Strahlenther Onkol. 2016;192(12):913-921.
- Wang J, Vyas S, Dang Q, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2013;86(5):885-891.
- Makishima H, Ishikawa H, Terunuma T, et al. Comparison of adverse effects of proton and X-ray chemoradiotherapy for esophageal cancer using an adaptive dose-volume histogram analysis. J Radiat Res. 2015;56(3):568-576.
- Lin SH, Merrell KW, Shen J, et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol. 2017;123(3):376-381.
- Hayman JA, Callahan JW, Herschtal A, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys. 2011;79(3):847-852.
- Deek MP, Benenati B, Kim S, et al. Thoracic vertebral body irradiation contributes to acute hematologic toxicity during chemoradiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;94(1):147-154.
- Lee J, Lin JB, Sun FJ, et al. Dosimetric predictors of acute haematological toxicity in oesophageal cancer patients treated with neoadjuvant chemoradiotherapy. Br J Radiol. 2016;89(1066):20160350.
- Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(5):1356-1365.
- Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(5):1431-1437.
- Warren S, Hurt CN, Crosby T, Partridge M, Hawkins. Potential of proton therapy to reduce acute hematologic toxicity in concurrent chemoradiation therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2017;99(3):729-737.
- Loi S, Sirtaine N. Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860-867.
- Zhang L, Conejo-Garcia JR, Katsaros, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203-213.
- Hyder J, Boggs DH, Hanna A, Suntharalingam M, Chuong MD. Changes in neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios during chemoradiation predict for survival and pathologic complete response in trimodality esophageal cancer patients. J Gastrointest Oncol. 2016;7(2):189-195.
- Davuluri R, Jiang W, Fang P, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(1):128-135.
- Shiraishi Y, Fang P, Xu C, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: a propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2017;S0167-8140(17)32751-2.
- Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. 2012;1(2):149-154.
- Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89(5):1084-1091.
- Welsh J, Settle SH, Amini A, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118(10):2632-2640.
- Marks LB, Ma J. Challenges in the clinical application of advanced technologies to reduce radiation-associated normal tissue injury. Int J Radiat Oncol Biol Phys. 2007;69(1):4-12.
- Settle S, Bucci MK, Palmer MB, et al. PET/CT fusion with treatment planning CT (TP CT) shows predominant pattern of locoregional failure in esophageal patients treated with chemoradiation (CRT) is in GTV. Int J Radiat Oncol Biol Phys. 2008;72(1):S72-S73.
- Kato K, Nakajima TE, Ito Y, et al. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for stage II-III esophageal carcinoma. Jpn J Clin Oncol. 2013;43(6):608-615.
- Fu WH, Wang LH, Zhou ZM, et al. Comparison of conformal and intensity-modulated techniques for simultaneous integrated boost radiotherapy of upper esophageal carcinoma. World J Gastroenterol. 2004;10(8):1098-1102.
- Nguyen GH, Murph MM, Chang JY. Cancer stem cell radioresistance and enrichment: where frontline radiation therapy may fail in lung and esophageal cancers. Cancers (Basel). 2011;3(1):1232-1252.
- Yu WW, Zhu ZF, Fu XL, et al. Simultaneous integrated boost intensity-modulated radiotherapy in esophageal carcinoma: early results of a phase II study. Strahlenther Onkol. 2014;190(11):979-986.
- Welsh JW, Sevedin SN, Allen PK, et al. Local control and toxicity of a simultaneous integrated boost for dose escalation in locally advanced esophageal cancer: interim results from a prospective phase I/II trial. J Thorac Oncol. 2017;12(2):375-382.
- Koyama S, Tsuji H, Yokota H, et al. Proton beam therapy for patients with esophageal carcinoma. Jpn J Clin Oncol. 1994;24(3):144-153.
- Koyama S, Tsujii H. Proton beam therapy with high-dose irradiation for superficial and advanced esophageal carcinomas. Clin Cancer Res. 2003;9(10 Pt 1):3571-3577.
- Mizumoto M, Sugahara S, Nakayama H, et al. Clinical results of proton-beam therapy for locoregionally advanced esophageal cancer. Strahlenther Onkol. 2010;186(9):482-488.
- Sugahara S, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for cancer of the esophagus. Int J Radiat Oncol Biol Phys. 2005;61(1):76-84.
- Gaspar LE, Winter K, Kocha WI, et al. A phase I/II study of external beam radiation, brachytherapy, and concurrent chemotherapy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207): final report. Cancer. 2000;88(5): 988-995.
- Okawa T, Dokiya T, Nishio M, Hishikawa Y, Morita K. Multi-institutional randomized trial of external radiotherapy with and without intraluminal brachytherapy for esophageal cancer in Japan. Japanese Society of Therapeutic Radiology and Oncology (JASTRO) Study Group. Int J Radiat Oncol Biol Phys.1999;45(3):623-628.
- Gaspar LE, Nag S, Herskovic A, Mantravadi R, Speiser B. American Brachytherapy Society (ABS) consensus guidelines for brachytherapy of esophageal cancer. Clinical Research Committee, American Brachytherapy Society, Philadelphia, PA. Int J Radiat Oncol Biol Phys.1997;38(1):127-132.
- Ishikawa H, Hashimoto T, Moriwaki T, et al. Proton beam therapy combined with concurrent chemotherapy for esophageal cancer. Anticancer Res. 2015;35(3);1757-1762.
- Reid TD, Davies IL, Mason J, et al. Stage for stage comparison of recurrence patterns after definitive chemoradiotherapy or surgery for oesophageal carcinoma. Clin Oncol (R Coll Radiol). 2012;24(9):617-624.
- Kato H, Fukuchi M, Miyazaki T, et al. Prediction of response to definitive chemoradiotherapy in esophageal cancer using positron emission tomography. Anticancer Res. 2007;27(4C):2627-2633.
- Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75(2):489-496.
Citation
Chuong, S B, M H, S A. Improving the therapeutic index for nonoperable esophageal cancer patients with modern radiation technologies. Appl Radiat Oncol. 2018;(3):7-14.
September 21, 2018