Hey, coach! Put me in! Improving the score in radiation oncology
Images

Imagine coaching a basketball game where you come up with a game plan but can’t change it or check the score until the game is over. This is basically what we do in radiation oncology. We pick a dose, deliver it in its entirety, and then reimage to see how we’ve done.
Just as a coach constantly evaluates a game and calls time-outs when the strategy isn’t working, we too must learn how to fittingly coach the game. To do this, we have to improve our understanding of the opponent, figure out how to best use our players, and check the score more often.
Scouting out the opponent
Our current approach to understanding our opponent typically involves figuring out how many players are on the team (ie, How big is the tumor?). In general, this has resulted in doses of 50-60 Gy for microscopic disease (a few players) and >70 Gy for gross disease (a lot of players). Our biggest advance in nuancing this has been defining different clinical target volume (CTV) dose levels and incorporating simultaneous integrated boosts. This development is essentially just an improvement in our ability to estimate the number of players on the team, but isn’t a game changer in terms of treatment success.
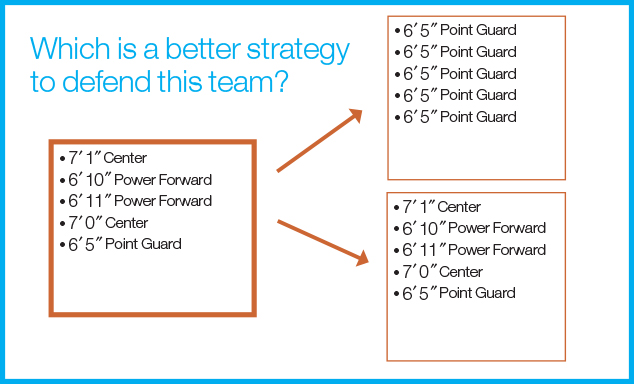
Other efforts have focused on boosting areas of higher standard uptake value (SUV) (ie, more players) on positron emission tomography (PET)-based studies, demonstrating that these areas are at higher risk of local failure. Work from the University of Michigan on non-small-cell lung cancer (NSCLC) demonstrates that mid-treatment PET can be used to modify volumes and allow for tumor dose escalation and dose reduction to normal tissues.1 This approach is being tested in clinical trials and will hopefully improve treatment outcomes, but still has a major weakness: It doesn’t address the identity of the team’s individual players and how this should impact treatment strategy. Isn’t it important to know if LeBron James and Kobe Bryant are on the team vs. a team comprised of bench warmers (Figure 1)? Perhaps one could argue that if you deliver enough dose then you will win the game regardless. But the problem with this approach is there are plenty of games that we still lose and, in some cases, do so quite badly. Understanding more about what makes the LeBron James’ of cancer so good—and how best to defend these top performers—is key to improving our winning percentage.
Understanding the team
In short, dose and volume are our star players. Advances in treatment machines and daily imaging have bulked up and improved the skill sets of these stars, but haven’t changed how best to use them. Generally, we push dose as high as we can without hurting adjacent normal tissues. When we reach toxicity limits, we consider delivering higher doses only to subvolumes. While moving dose around to different volumes looks pretty on the treatment planning station, it’s akin to watching the Harlem Globetrotters perform fancy tricks before they shoot a basket. It may be fun to watch, but it detracts from the game. We ultimately need to more adeptly use dose and volume. It’s possible that hypofractionation (not related to adaptive planning) is better for defending only certain players, while a combination of standard fractionation and hypofractionation is best for others. In fact, using the maximal dose may not be the best strategy at all. Radiation results in significant genomic changes in tumors and can change the expression pattern of tumors to be more susceptible to certain pathway inhibitors. We must remember that multiple targeted agents (not adaptive strategies) are entering the draft each year and we need to recruit them to join our team. It’s important to think about which of these players are superstars and will synergize with radiation, and which ones we don’t need. As sexy new players like programmed cell death 1 inhibitors enter the draft, we must scrutinize their real value and not be wooed by the hype. In addition, we must determine how best to integrate our powerful roster of players with any newcomers (Figure 2).
HIV-positive oropharynx cancer presents an interesting example in that we are finding dose de-escalation may be a reasonable strategy. However, larger therapeutic gains might be possible with reductions/omission of systemic therapy in favor of targeted agents, rather than just pursuing modest reductions in radiation dose.
What’s the score?
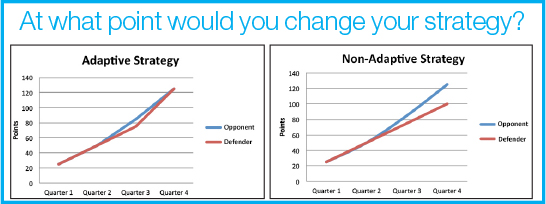
To improve our understanding of how we’re doing, we must watch the game in real time (Figure 3). We need to adapt and learn during the game rather than reflect on why we lost after the game is over. MRI is an important component of helping us see the game, as it provides superior soft-tissue definition, functional information, and no additional radiation exposure to the patient. Integrating serial MRI scans and biopsy as tools to predict treatment response has been demonstrated in the investigation of serial studies to predict a therapeutic response with the imaging and molecular analysis (I-SPY) program in breast cancer. ISPY-1 was a collaboration between the National Cancer Institute Specialized Programs of Research Excellence (NCI SPOREs), the American College of Radiology Imaging Network (ACRIN), the Cancer and Leukemia Group B (CALGB), and the NCI Center for Biomedical Informatics and Information Technology (CBIIT). It demonstrated that disparate disciplines could come together to integrate biomarkers and imaging data at multiple time points during treatment to help predict pathologic complete response after neoadjuvant chemotherapy in breast cancer.
The follow-up ISPY-2 trial is an ambitious replacement trial that incorporates an adaptive clinical trial design.2 It has two arms: one in which patients receive standard neoadjuvant chemotherapy, and one in which patients receive standard chemotherapy plus 1 of 5 new drugs. Patients will have 3 biopsies and 4 MRIs performed during the course of neoadjuvant chemotherapy (Time 0, 3 weeks, 12 weeks, and prior to surgery for the additional MRI). The primary endpoint of the study is pathologic complete response. Correlations between treatment response with imaging and biomarker changes will be made. Using the 4 MRIs and multiple biopsies will allow investigators to observe the game essentially every quarter.
While this model is appealing, the negative findings from the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO) trial presented in June at the American Society of Clinical Oncology (ASCO) meeting in Chicago, call into question whether pathologic complete response is an appropriate marker. The combination of Lapatinib and Trastuzumab increased pathologic complete response in the neoadjuvant setting (NeoALTTO); however, these positive findings were not reproduced in the adjuvant setting.
While improvements in adaptive trial design are still needed, there are opportunities to develop such trials in radiation oncology. One example is a high-risk prostate cancer protocol that is open to accrual at the University of California Los Angeles (UCLA). As with the neoadjuvant chemotherapy model in breast cancer, high-risk patients receive neoadjuvant androgen deprivation therapy (ADT) for 2 months before initiating radiation therapy. Data from the prostate literature suggests that a patient’s initial response to neoadjuvant ADT as measured by the patient’s prostate-specific antigen (PSA) before starting RT (ie, < 0.5), is ultimately predictive of patient outcome. This provides an early biomarker before the start of radiation therapy (RT), which could potentially stratify patients. Multiparametric MRI could be performed before starting ADT. Fiducial marker seeds could be placed in areas outside of MRI targets (to avoid interference on functional imaging) before starting ADT as well. During the placement of the marker seeds, an ultrasound/MRI fusion technique could be used to take a couple cores from the index lesion as determined from the MRI. The patient could then have his PSA measured again in 2 months with another multi-parametric MRI exam before starting a brachytherapy implant as a boost.
At the time of brachytherapy, another biopsy could be performed in the operating room on the index lesion. The information gleaned from such a study could identify MRI and/or genomic features that could predict which men are not likely to achieve a low PSA value prior to starting radiation treatment. These men could be identified to perhaps try newer anti-androgen therapies or dose escalation to the dominant site of disease. Alternatively, for patients who do achieve a low PSA nadir, we may find that they are ultimately not destined to develop metastatic disease, and perhaps could avoid prolonged androgen deprivation.
Where is everyone on the court?
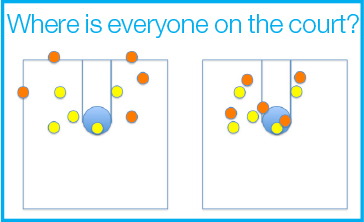
When we treat patients, we place a margin around our target to ensure we don’t miss secondary to set-up error and/or organ motion. These margins are analogous to placing defenders in areas that are out of bounds (Figure 4). Daily image guidance has improved our ability to tighten these margins and minimizes how much area outside the line we are defending. Aside from not defending areas unnecessarily, you also want to know where the players are that you’re defending on the court—a distribution that changes over the course of the game. Daily MRI imaging allows one to see how the initial distribution (ie, a 5 cm tumor) is changing over the course of treatment, as well as providing the opportunity to shrink the field, if appropriate. Ultimately, determining “appropriate” changes in treatment fields must be investigated using prospective adaptive trials, since over-adapting is also possible and could increase failures. If all players are initially spread out and occupy all the space from the baseline to the half-court line, but then all players move to between the baseline and the free-throw line, you would want to know this and move players accordingly. Two choices come with this decision: real time on-line adaptive planning and off-line adaptive planning. With the former, the defensive players can tell where the offensive players are and move to cover them. With off-line adaptive planning, the defensive players can’t tell where the offensive players are, but the coach can see changes, call a time out and rearrange the defense quickly. The problem with this approach is time-outs are limited, so you must take them at the right times.
The other component of this is deciding whether to play man-to-man defense or zone. We typically play a zone defense in which we cover a large area (planning target volume). The problem is that our zone coverage isn’t set up right. To ensure you don’t surrender easy points and limit “points in the paint,” teams often have 2 players at the free-throw line and 3 closer to the basket. Our standard approach is to have a homogeneous distribution throughout the whole target. This doesn’t make sense when you need to cover higher risk areas on the court. With functional MRI imaging, you may be able to determine if LeBron James is moving around during the course of treatment. Maybe at the beginning he’s at the free-throw line, but toward the middle of treatment he moves behind the 3-point line. Depending on the situation, you may also want to double team LeBron James while covering the rest of the court by a zone. To make this call, you must see what’s happening.
Final thoughts
To truly adapt a treatment plan, one must understand what is going on and how the patient is responding. Advances in MRI imaging and MRI treatment planning will allow us to image a patient daily and help us accomplish these goals.
We must stop coaching with our eyes shut. If we watch the game as it unfolds, we can potentially call a time out when needed, and put the right players in to turn the game around. The ViewRay (Oakwood Village, Ohio) MRI-guided radiation therapy system presents an exciting new frontier toward this end. The ability to view a patient’s anatomy using MRI imaging in real-time during treatment is a major step forward. This treatment platform allows us to pursue adaptive planning in a way we could only have dreamed of a decade ago. The first patient using the ViewRay system was treated this year, and we are excited to start adapting this technology for winning results.
References
- Feng M, Kong FM, Gross M et al. Using fluorodeoxyglucose positron emission tomorgraphy to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int J Radiat Oncol Biol Phys. 2009;15;73(4):1228-34.
- Barker AD, Sigman CC, Kellof GJ et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86(1):97-100.