Hemangiopericytoma of the intra- and suprasellar regions
Images
CASE SUMMARY
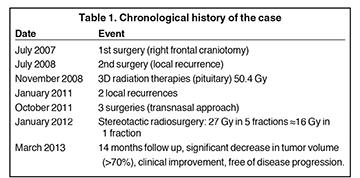
A 72-year-old woman with a one-month history of blurred vision, diplopia, left ptosis, dizziness and limited walking ability was referred by her neurosurgeon to the Radiation Oncology-Radiosurgery Department of Clínica Abreu. A cranial magnetic resonance imaging (MRI) scan revealed a sellar mass, and the patient underwent a right frontal craniotomy in July 2007. The surgical pathology revealed an intra- and suprasellar hemangiopericytoma.
One year later, the patient underwent a second right frontal craniotomy because of local recurrence. Posteriorly, the patient received adjuvant 3-dimensional radiotherapy, receiving a total dose of 50.4 Gy with conventional fractionation of 1.8 Gy/day, using photons of 18 Mv. Approximately 3 years later, a follow-up cranial MRI reported a proliferative sellar process with extension to the suprasellar region, the left cavernous sinus, and the sphenoid sinus, measuring 27 mm × 25 mm.
An MRI scan of the brain in August 2011 revealed the same proliferative sellar lesion, measuring 34 mm × 26 mm × 39 mm, withsuprasellar extension, obliterating the suprachiasmatic cistern, exerting discrete mass effect on the optic chiasm and gently contacting the left rectus gyrus, as well as invading the left cavernous sinus, sphenoid sinus, and clivus, and obliterating the prepontine cistern. ACT angiography performed at this time showed left carotid compromise surrounded by the lesion.
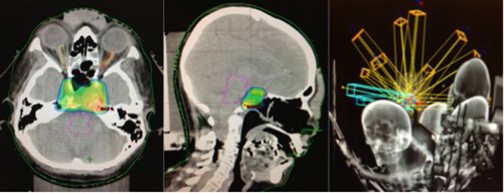
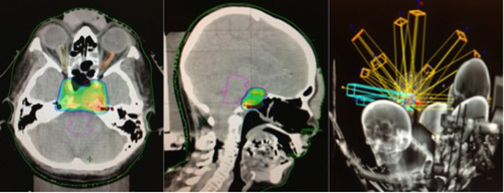
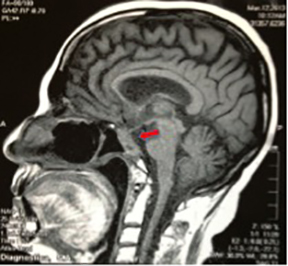
The patient underwent a third surgery, but this time with a transnasal approach. A postsurgical MRI scan showed an intra- and suprasellar expansive lesion with slight increase of volume in the described lesion, compared with previous studies (Figure 1). Before treatment planning, visual campimetry revealed a bilateral hemianopsia. After immobilizing the patient with a thermoplastic Byte Blockmask, we performed brain MRI and CT scans with and without contrast, with subsequent image fusion and planning in the HeliosEclipse® system.
The patient underwent stereotactic radiosurgery in a Clinac 21 iX linear accelerator (Varian), setting the target with one isocenter and 9fields, with 27 Gy prescribed in 5 sessions with a dose-per-fraction of 5.4 Gy/day. This was radiobiologically equivalent to one treatment session at 16 Gy using an α / β of 10 (Figure 2). The patient tolerated the treatment without complications, and 3 months’ post-treatment demonstrated significant improvement on her left eyelid movement. At 6 months, an MRI scan showed appreciable tumor volume decrease compared with previous studies; at 1 year post-treatment, a cranial MRI demonstrated a 70% decrease in tumor volume compared to pretreatment lesion volume (Figure 3). At 14 months, our patient completely recovered her left eyelid mobility and experienced significant improvement in visual acuity and stability of walking ability.
DIAGNOSIS
Hemangiopericytoma of the intra- and suprasellar regions
DISCUSSION
Hemangiopericytoma (HPC) is a rare vascular tumor that arises from the pericytes of Zimmerman, is associated with the capillary walls, and can appear anywhere in the body. HPC’s appearance in the central nervous system (CNS) is rare, accounting for only 0.4% of primary CNS tumors; only about 137 cases are reported in the literature. Hemangiopericytomas of the sellar and parasellar regions are extremely rare and represent only 1% of all primary intracranial hemangiopericytomas, with only 6 cases reported worldwide. Although initially it was believed that HPC was a variant of meningioma (angioblastic meningioma) it has been recognized as a distinct entity with different clinical, immunohistochemical and ultrastructural characteristics.
Historically, hemangiopericytomas have been wrongfully grouped with other neoplasms. In 1938, Cushing and Eisenhard rated the HPC as one of 3 variants of angioblastic meningioma. In 1942, Stout and Murray first described hemangiopericytoma as a malignant, separated neoplasm of capillary pericytes with different pathologic features, and Begg and Garret in 1954 reported the first case of intracranial HPC.
It is well known and accepted that hemangiopericytomas and meningiomas originate from multipotential precursor cells, the difference being that hemangiopericytomas originate from pericytes, not from arachnoid cells. Ultrastructurally, the presence of a basementmembrane and the absence of desmosomal attachments distinguish hemangiopericytomas from meningiomas, so definitive diagnosis is based on histopathological, ultrastructural, and immunohistochemical differences.4,6 Meningiomas have tumor markers such as keratin and epithelial membrane antigen, whereas hemangiopericytomas, being of mesodermal origin, do not.6
The term “meningeal hemangiopericytoma” is commonly used because most hemangiopericytomas arise from the meninges (where pericytes also exist), but they may also occur in the brain parenchyma in a pure form, without the meningeal component. HPC is usually seen in adults, and compared with meningioma, it occurs more often in young men (mean age at diagnosis is 43 years), exhibits faster growth and tends to recur and metastasize, commonly outside the CNS in such areas as the bone, liver and lung. Recurrence depends on the extent of resection, tumor volume,and histopathological aggressiveness.6 In the literature, recurrence rates at 5, 10, and 15 years are 65%, 76%, and 87%, respectively, with an overall survival rate of approximately 43% at 15 years.1,5,12 Hemangiopericytomas located in the sellar, suprasellar, and parasellar regions are extremely rare; only 6 cases are reported in the literature.4,12 They can mimic pituitary adenomas, with similar symptoms. In all reported cases patients have shown decreased visual acuity; 4 were diagnosed with bilateral hemianopsia, as was our patient.
Although hemangiopericytomas are more common in men, sellar lesions are more common in women.4,12 These neoplasms usually present as discrete masses, but they may invade adjacent structures, such as in our case, where the lesion extended into the left cavernous sinus, sphenoid sinus, clivus, and even the left carotid artery. Indeed, the biologically aggressive behavior of these tumors is another factor that can limit treatment.
Radiation therapy is used in almost all intracranial hemangiopericytomas, especially unresectable hemangiopericytomas. Ecker and colleagues reported 38 HPCs treated with RT with or without SRS and concluded that SRS in recurrent disease contributed to improved survival.1,10 In 1993, Coffey and colleagues published the first preliminary report of SRS for HPC, with a total of 11 lesions treated in 5patients who had undergone previous surgical resection. Of the 11 tumors, 9 shrank or remained stable at an average of 14.8 months.1 In a recent series of 20 patients, a higher dose of 14 Gy was significantly associated with improved progression-free survival (average of 79.4 months at dose >14 Gy vs. 45.2 months at dose <14 Gy).1
At this writing, our patient is experiencing progression-free survival of 14 months and showing no evidence of extracranial metastases
CONCLUSION
Hemangiopericytoma is an aggressive vascular tumor that tends to recur and metastasize, even after total resection. They are extremely rare in the sellar and parasellar regions and they can often mimic pituitary adenomas.
Postoperative radiotherapy is mandatory, even when complete tumor removal is achieved, especially when the tumor extends to adjacent structures, significantly reducing local recurrence. Stereotactic radiosurgery is of great value for recurrences in the central nervous system, even in previously irradiated patients or after tumor multiple resections.
REFERENCES
- Kano H, Niranjan A, Kondziolka D, et al. Stereotactic radiosurgery after resection of intracranial hemangiopericytomas, Int J Radiation Oncology Biol Phys. 2008;72:13331339.
- Veeravagu A, Jiang B, Patil CG, et al. CyberKnife stereotactic radiosurgery for recurrent, metastatic, and residual hemangiopericytomas, J Hemat Oncol. 2011;4:26.
- Spatola C, Privitera G. Recurrent intracranial hemangiopericytomas with extracranial and multiple metastases, case report and review of the literature. Servizio di Radioterapia, Policlinico Universitario di Catania, Italy, Tumori, 2004;90:265-268.
- S Safavi-Abbasi, I Feiz-Erfan, RE Bristol, WL White, Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona. Hemangiopericytoma of the parasellar region: Case report and review of the literature. Barrow Quarterly. 2005; 21:4.
- Kim JH, Jung W, Kim Y-S, et al. Meningeal hemangiopericytomas: Long-term outcome and biological behavior. Surg Neurol. 2003;59:47-54.
- Mejía M, Cabezas C, Teodoro E, Francisco S. Hemangiopericitomas: Presentación de un caso clínico. Binass, Revista Neuroeje vl4n2.
- Olson C, Yen CP, Schlesinger D, Sheehan J. Radiosurgery for intracranial hemangiopericytomas: Outcome after initial and repeat GammaKnife surgery, J Neurosurg. 2010;112:133-139.
- Coppa ND, Raper DM, Zhang Y. Treatment of malignant tumors of the skull base with multi-session radiosurgery, J Hematol Oncol. 2009;2:16. doi:10.1186/1756-8722-2-16.
- Thiringer JK, Costantino PD, Houston G. Sinonasal hemangiopericytoma: Case report and literature review, Skull Base Surgery. 1995;5.
- Ecker RD, Marsh WR, Pollock BE, et al. Hemangiopericytoma in the central nervous system: Treatment, pathological features, and long-term follow up in 38 patients. J Neurosurg. 2003;98: 1182–1187.
- Perez & Brady’s, Principles & Practice of Radiation Oncology. 5th Edition; Lippincott, Philadelphia, Pa: 746-747.
- Das P, Haresh KP, Suri V, et al. Malignant hemangiopericytoma of pituitary fossa. Indian J Pathol Microbiol. 2010;53:109-11.
- Han MH, Cho YD, Young-Don Kim, Kim DH. Recurrent sellar and suprasellar hemangiopericytoma, J Korean Neurosurg Soc. 2007;41:425-428.