FLASH Stance — Updates in Ultrahigh Dose Rate Radiation Therapy
Images
FLASH radiation therapy (FLASH- RT), a technique that delivers an ultrahigh dose of radiation in 1 second or less, is being heralded as a promising treatment option that could potentially transform cancer care.
In the 1960s, early experiments found reduced damage and greater variability in noncancerous mammalian cells irradiated at very high dose rates compared with conventional dose rates.1 Piquing more recent interest was the key factor that cancerous tissue does not observe the saturation effect with FLASH-RT.
“It was pretty evident from very early that this needed to be much more than just a technology foray,” says Agam Sharda, vice president of FLASH Solutions at Varian, which is examining FLASH-RT as holistic therapy. “It could affect tissue in a different way than radiation typically does.”
FLASH-RT produces a phenomenon called the FLASH effect, which provides tumor control and minimal toxicity to normal surrounding tissues. While underlying mechanisms behind the FLASH effect are not fully known, two primary hypotheses have emerged. One is that an immune response contributes to the FLASH effect.
“There are indications that delivering the dose so quickly has an effect on the immune system,” says Kristoffer Petersson, PhD, medical research council investigator and group leader – FLASH Radiation, Department of Oncology, Medical Sciences Division, University of Oxford. “There has to be something else also contributing, since we still see a FLASH sparing effect in immunocompromised animals.”
The second hypothesis centers on oxygen depletion in the cells, in which the ultrahigh doses produce a period of hypoxia that does not seem to change tumor radioresistance. However, in normal tissue it leads to large, rapid increases in tissue radioresistance, thereby protecting the normal tissue.2
“We’ve seen in vitro and in vivo that when you modify the oxygen content you get a modified effect,” says Dr. Petersson, who is investigating the biology and underlying mechanisms behind the FLASH effect. “But we also have very recent studies now showing that we have an effect at low doses in normal conditions where you wouldn’t expect oxygen depletion to play a role.”
A recent study demonstrated in vitro that after a certain dose level, cells exposed to FLASH irradiation begin to behave in a hypoxic manner. In this study, there was a clear FLASH effect that relied on oxygen concentration.3
David Gladstone, ScD, DABMP, FAAPM, chief of clinical physics at Dartmouth-Hitchcock’s Norris Cotton Cancer Center, is leading a group that has also studied oxygen depletion in mice under FLASH conditions.4 “So far, we have not measured a change in oxygen sufficient to explain the clinical effects that are seen in terms of reduction of damage to the normal tissues,” he says. “That’s not to say that oxygen isn’t involved, but it’s not the entire story.”
The Dartmouth group is also conducting a genetic analysis of irradiated tissues, comparing FLASH to conventional doses, looking for molecular markers that could shed light on what part of the process is changing.
Another area under exploration is how FLASH may work in tandem with other treatment modalities, such as immunotherapy and chemotherapy. According to Swati Girdhani, director of Research Collaborations at IBA, FLASH would enable faster and shorter treatments, reducing the volume of blood irradiated, and lower subsequent reduced killing of circulating immune cells, including lymphocytes, the main mediator of immune response to cancers.5
FLASH-RT may also expand the reach and indications for RT treatment, says Girdhani. “With FLASH therapy, if we can reduce normal tissue toxicity, it opens the potential to perform dose escalation on radioresistant tumors like glioblastomas and radiation treatment of tumors surrounded by radiosensitive tissue like ovarian cancers.”
There is also a radical-radical interaction that has an effect, says Dr. Petersson. “When you irradiate, you generate radicals that can damage DNA. With FLASH, you put in so much dose at one time that the concentration of the radicals formed is greater, with a much higher probability of these to interact with each other before they damage DNA. So that could be one explanation: that these radicals that are created when the radiation interacts in and around the cell result in a concentration that is so high that the effect on DNA may be lower than when using lower dose rates.”
Dr. Petersson found that current radiation dose detectors for beam monitoring decrease in efficiency down to just a few percent.
“When you go to an ultrahigh dose rate that lasts a few microseconds, it is much more challenging to get a good measurement of the dose that you are delivering and also to control the delivery,” Dr. Petersson explains. “In my opinion, FLASH will be introduced in the clinic as a hypofractionated treatment, possibly at even larger volumes than we normally do now.”
First Human Clinical Trial and Treatment
In November 2020, the Cincinnati Children’s/UC Health Proton Therapy Center began the first clinical trial and human treatment using FLASH-RT. The Feasibility Study of FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases (FAST-01) is sponsored by Varian and will include up to 10 patients ages 18 years or older who have up to three painful bone metastases in the extremities.
The Proton Therapy Center houses a $24 million research facility with a 300-ton gantry that mirrors the dosimetry and operation of the clinical gantry. Having combined clinical and research centers under one roof allows for simulated treatments in the animal models to translate right to patients, says John Perentesis, MD, director of the Division of Oncology and Cancer Programs at Cincinnati Children’s.
“With FLASH, we were able to do in vitro studies on what happens in the test tube on cancer cells and then, even more importantly, take it to the next dimension in terms of side effects in animals and then in animals with cancer,” he says. “That pre-clinical data supported the hypothesis that FLASH radiation of the extremities … was less toxic.”
Study participants will only include patients with arm or leg bone metastases so if adverse side effects arise, critical organs or structures will not be affected. “We are looking at whether or not we can use FLASH to deliver radiation and have fewer side effects in patients,” says John C. Breneman, MD, medical director of the Proton Therapy Center on the Liberty Campus of Cincinnati Children’s. “With FLASH therapy, the preclinical data in the animal studies show that you can have efficacy in treating tumors but with fewer side effects.”
Future investigations at the Proton Therapy Center include pre-clinical studies comparing FLASH-RT with proton therapy in thorax and lung cancer in terms of induction of pulmonary fibrosis and in efficacy of tumor death, Dr. Perentesis says. There is also interest in chest/thoracic and brain cancers, particularly comparing efficacy with tissue toxicity.
Modifying the Accelerator
Clinical linear accelerators can be modified to deliver FLASH-RT, and throughout much of his career, Dr. Gladstone has conducted experiments on modified linear accelerators. Examples include adding one of the first electronic portal imaging devices on a linac prior to commercial development6 and gating an accelerator to the cardiac cycle, demonstrating a mechanism to spare the heart from radiation damage.7
To create a high-intensity beam from a clinical linear accelerator, Dr. Gladstone worked with a team of medical physicists and biomedical engineers at Dartmouth College and Dartmouth-Hitchcock Medical Center. They programmed an older accelerator that had limited clinical use to deliver a pristine electron beam by pulling the x-ray target out of the beam’s path to achieve the desired dose rate. To perform these experiments, the team developed a new optical technique to measure the dose rate and dose distribution that would enable acquisition of a three-dimensional dosimetry using a single pulse of radiation from the linac.8
Dr. Gladstone and his colleagues then tackled beam control, achieving approximately 1 Gy of dose per pulse. “We have control over the machine by counting pulses,” he explains. “We want to integrate the dose per pulse as they come through — like any normal accelerator using an ionization chamber — to increase the precision of dose delivery to fractional pulse levels.”
Using a FLASH beam, three animals from the community have been treated at Dartmouth-Hitchcock on the modified linac under an NCI-funded spontaneous animal tumor grant.
“We have been able to safely use uncharacteristically high RT doses in our spontaneous canine cancer FLASH patients,” Dr. Gladstone says. “Although the total dose has been spread temporally over a longer period than typically used in clinical medicine, the dose is approximately 30% higher than what we would generally believe acceptable.”
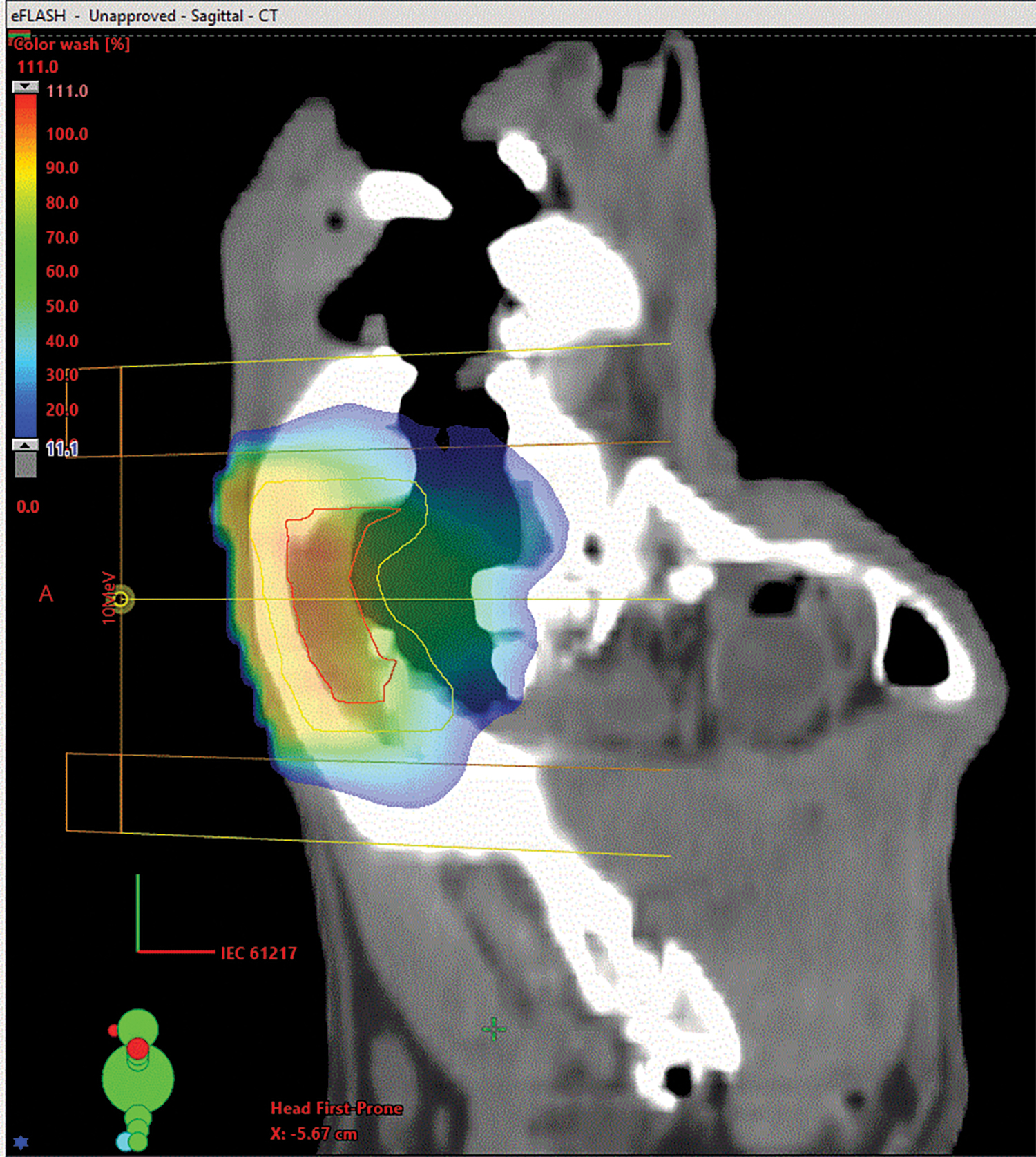
While two of the canine tumors treated with FLASH were oral melanoma and soft-tissue sarcoma, which are historically incurable with conventional surgery and radiation, both dogs remain in full health remission 9 and 12 months post RT, he adds. While superficial skin and mucosa damage was noted, healing is progressing well without additional support. In the oral melanoma case, the dog has thrived. (Figure 1 shows a canine treatment plan using FLASH-RT.)
“It’s really going to be fascinating work in the years ahead to try to bring this to humans to increase the therapeutic ratio and get better outcomes both in terms of tumor control and reducing normal tissue toxicities,” he adds.
The Technology Behind FLASH-RT
Three proton therapy manufacturers are developing FLASH-RT. At Mevion Medical Systems, the FLASH delivery capability is being pursued with the company’s pencil-beam scanning system. The architecture with a downstream range shifter keeps high dose rates at all energies for different delivery depths, explains Townsend Zwart, vice president of Advanced Development at Mevion. The company’s proton multileaf collimator with an adaptive aperture can sculpt sharp edges that may be useful for constructing large volumes.
The expectation is that components will be added to existing proton therapy systems to enable FLASH-RT — from dosimetry to accurately measure the short, intense pulses of radiation, to patient positioning equipment. Zwart believes positioning and the errors allowed in treatment planning will need to improve across the field to allow for clinical use of FLASH-RT.
FLASH delivery will also make motion management much more attractable, says Zwart. “People don’t move much inside a quarter of a second,” he says. “It will make setup and image guidance before delivery that much more critical.”
Plus, with the expectation that FLASH may lead to more hypofractionated treatments, Zwart sees an opportunity to increase the utilization of proton therapy systems to treat more patients and provide greater access to proton therapy.
Regarding standard dose rate for FLASH- RT, while 40 Gy per second seems common in current studies, Zwart says Mevion is preparing to hit higher dose rate levels if research shows higher is better.
At Varian, the company is looking at FLASH holistically, focusing on the role of technology throughout the entire patient experience.
“FLASH is a very promising therapy that, if it comes to pass, will benefit a lot of patients,” says Agam Sharda, vice president of FLASH Solutions at Varian. “But these current machines were not really validated to do this. So Varian is being extra cautious to make sure we develop the tools, mechanisms and technologies that will maximize safety for all involved.”
Although Varian provides various radiation therapy technologies potentially adaptable for FLASH—proton, photon, electron and brachytherapy—Sharda believes protons offer the greatest initial potential.
“We are convinced that the fastest, most effective and efficient way of giving FLASH to deep-seated tumors is via proton therapy,” says Sharda. “Electrons are better suited for superficial targets, so there is fantastic complementarity between electron FLASH and proton FLASH.”
To enable FLASH proton RT required an almost complete redesign of the control system of the beam delivery mechanism to count the rapid rate of protons, he says.
However, Sharda sees photon FLASH requiring greater engineering investments and innovation, positioning it behind proton and electron FLASH development.
Varian is also looking at electron FLASH in the same way it has pursued proton FLASH over the last four years. In the near future, Varian will provide electron FLASH research capabilities to interested linac customers.
In treatment planning, the key parameter is the dose rate being delivered. As such, in addition to looking at the spatial distribution of the dose rate, treatment planning for FLASH-RT must also consider the temporal distribution of dose. “We have to start thinking about a patient’s treatment plan as a dose-rate-volume histogram in addition to dose-volume histogram,” Sharda says.
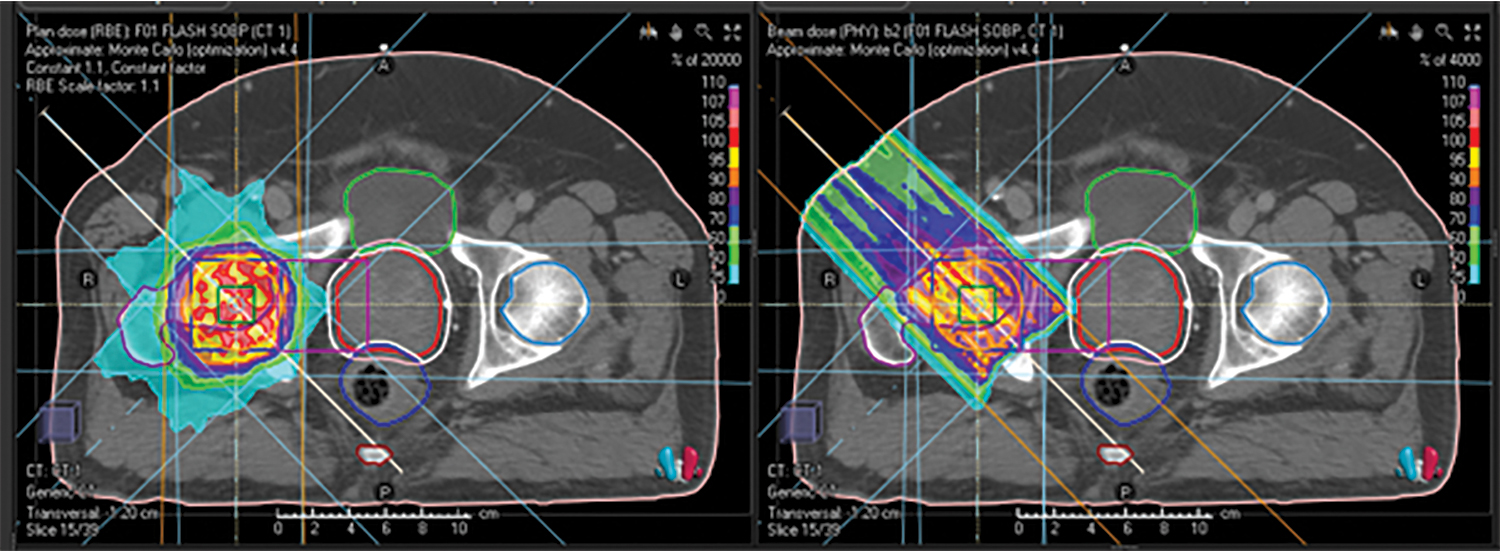
At IBA, the company is pursuing conformal FLASH, which uses FLASH dose rates as well as the Bragg peak, says Nicolas Denef, emerging therapies director at IBA. By combining a single layer of pencil-beam scanning irradiation with a field-specific filter, the technology may enable FLASH irradiations that also stay conformal to the target, thereby combining FLASH and the superior dose conformality of proton beams. (See example in Figure 2.)
However, more work remains before initiating clinical trials that use the Bragg peak of protons in FLASH-RT. In terms of existing IBA accelerators, any future FLASH capability will likely be provided as an upgrade. While the primary focus is on proton therapy, IBA’s subsidiary Normandy Hadrontherapy is building a carbon therapy system that may have the capability to provide FLASH.
Proceeding With Caution
As progress continues, avoiding haste and unnecessary risks is essential. With FLASH, clinicians are not afforded the same time they have with conventional RT to react and adjust to issues that arise during treatment.
“We want to make sure that with FLASH we have the same level of quality assurance that we have with 30 treatments as with one treatment that will take a fraction of a second,” says Denef. “We also need to have high precision electronics that ensure FLASH is safely delivered.”
While RT has focused on improving the physics of beam delivery for years, FLASH is part of a trend of better understanding and optimizing the biology of ionized particles, Denef says.
“The biology studies currently being carried out may help us understand the molecular pathways generated by FLASH radiation, and potentially lead to new treatments in the future,” he says.
References
- Durante M, Bräuer-Krisch E, Hill M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br J Radiol. 2018;91(1082):20170628. doi:10.1259/bjr.20170628
- Wilson JD, Hammond EM, Higgins GS, Petersson K. Ultra-high dose rate (FLASH) radiotherapy: silver bullet or fool’s gold? [published correction in Front Oncol. 2020 Feb 25;10:210]. Front Oncol. 2020;9:1563. Published 2020 Jan 17. doi:10.3389/fonc.2019.01563
- Adrian G, Konradsson E, Lempart M, Bäck S, Ceberg C, Petersson K. The FLASH effect depends on oxygen concentration. Br J Radiol. 2020;93(1106):20190702. doi: 10.1259/bjr.20190702
- Cao X, Zhang R, Esipova TV, et al. Quantification of oxygen depletion during FLASH irradiation in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2021;9:S0360-3016(21)00358-8. doi:10.1016/j.ijrobp.2021.03.056
- Jin JY, Gu A, Wang W, Oleinick NL, Machtay M, Spring Kong FM. Ultra-high dose rate effect on circulating immune cells: a potential mechanism for FLASH effect? Radiother Oncol. 2020;149:55-62. doi:10.1016/j.radonc.2020.04.054
- Gladstone DJ, van Herk M, Chin LM. Verification of patient setup before total body irradiation (TBI) using an electronic portal imaging device (EPID). Int J Radiat Oncol Biol Phys. 1993;27(2):449-454.
- Gladstone DJ, Flanagan MF, Southworth JB, et al. Radiation-induced cardiomyopathy as a function of radiation beam gating to the cardiac cycle. Phys Med Biol. 2004;49:1475-1482.
- Rahman M, Ashraf MR, Zhang R, et al. Electron FLASH delivery at treatment room isocenter for efficient reversible conversion of a clinical LINAC. Int J Radiat Oncol Biol Phys. 2021;1:S0360-3016(21)00024-9. doi:10.1016/j.ijrobp.2021.01.011
Citation
MB M. FLASH Stance — Updates in Ultrahigh Dose Rate Radiation Therapy. Appl Radiat Oncol. 2021;(2):38-41.
July 27, 2021