Equipment Launches and Updates from ASTRO 2020
Images

Hosted virtually at the Miami Convention Center in October, the 62nd annual meeting of the American Society for Radiation Oncology (ASTRO) featured the theme “Global Oncology: Radiation Therapy in a Changing World,” and shared timely COVID-19 experiences as well as an array of new attendee options, from Storytelling Sessions and ASTRO Voices, to Master Classes providing a deeper dive on topics.
The meeting also showcased a robust lineup of keynotes, posters, scientific sessions, networking zones, SA-CME opportunities and more, as well as updates from roughly 100 vendors in the virtual exhibit hall. Following is a recap of equipment news and vendor highlights that continue to shape the path of radiation therapy treatment.
Palo Alto, California-based Varian presented innovations that deliver more data-driven insights to improve efficiencies and outcomes, making cancer care more personalized. Presentations included data from the use of Varian’s adaptive radiation therapy system, Ethos, for lung, prostate, bladder, upper abdomen, head and neck cancers, and first-look data from the recently formed artificial intelligence (AI)-driven Adaptive Intelligence Consortium.
“Ethos is the world’s first AI-powered linear accelerator. It segments both the tumor volume and the associated surrounding healthy tissue to personalize and adapt treatment so therapy can be focused on the tumor and avoid the healthy tissue,” said Chris Toth, president and COO of Varian. “That’s the Holy Grail of what we’ve been chasing for two decades within radiation oncology, so we can personalize and adapt treatments in a standard time slot.”
The company also is transitioning its training approach to on-demand resources and tools with Varian Think, an online repository for individualized educational content. “We’ve doubled the investment in this area to support our customers not only with the best technology, but also the best service and support,” said Toth.
The company’s remote connectivity and telehealth telemedicine support services help clinicians keep cancer patients safe, especially during COVID-19. “This highlights the flexibility of our software applications and how we can keep centers and clinics powered in the midst of the pandemic. It shows how we make sure that that patient care doesn’t skip a beat,” he said.
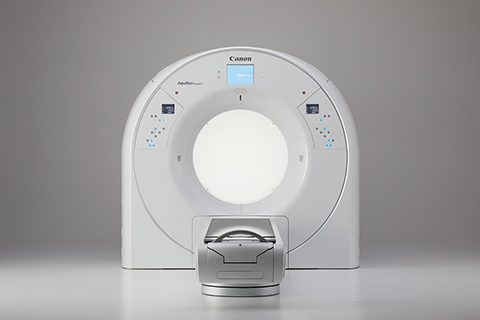
Empowering clinicians to see more during radiation therapy planning, Canon Medical Systems USA, Inc. introduced the Aquilion Exceed LB CT system (pending 510[k] clearance), (Figure 1) an AI-powered premium scanner featuring the industry’s largest bore and widest field of view. It enables better contouring using AI with sharp, clear and distinct images from Canon’s Advanced intelligent Clear-IQ Engine (AiCE) deep-learning reconstruction technology.
“We’re excited to release a system that has AiCE DLR technology with a huge bore opening. This is the first system in the radiation oncology space that has deep-learning reconstruction technology,” said Erin Angel, managing director of Canon’s CT Business Unit. “It improves low-contrast detectability and the visibility of small details so clinicians can make sure the contours of low-contrast lesions are accurate.”
The Aquilion Exceed LB delivers accuracy in complex simulations with a 90-cm bore opening, edge-to-edge extended 90-cm field-of-view reconstruction, and the widest detector coverage (4 cm) in radiation oncology. “It’s a spec monster. In every aspect of the system, we’ve surpassed what the industry expects in a radiation oncology system,” said Dhruv Mehta, leader of strategic development for CT at Canon. “In radiation oncology, it’s all about patient positioning. With a 90-cm bore, you’re able to ensure accurate positioning of the patient, with AI to improve image quality, because that’s the one thing that will matter for every single patient on every single study.”
Reinforcing its commitment to innovation, GE Healthcare rolled out a roadmap for its portfolio of scanner solutions, including the new Revolution Apex ultrapremium CTand the Discovery RT CT scanner for radiation therapy planning.
Revolution Apex combines a powerful new imaging chain with TrueFidelity CT images created by deep-learning image reconstruction. It gives clinicians access to outstanding coverage, spatial resolution, temporal resolution and spectral imaging capabilities in one system, with the power of the Quantix 160 tube for excellent image quality.
Discovery RT offers a streamlined workflow and submillimetric images with motion and metal artifacts significantly reduced.
On the simulation side, the company presented AIR Recon DL (not CE marked), a deep-learning-based reconstruction algorithm that shortens scan times while delivering better image quality across all anatomies. Developed on GE Healthcare’s Edison intelligence platform, the technology generates AIR Recon DL images in real-time at the operator’s console.
“By leveraging AI technology, AIR Recon DL helps improve signal-to-noise ratio for better delineation. It supports radiation therapy planning by precisely targeting tumors and minimizing collateral damage for efficacy and optimization of the outcome,” said Ben Newton, general manager, oncology business for GE Healthcare.
The company complements those technologies with digital solutions, such as AdvantageSim MD, an integrated simulation and localization software suite that helps improve and streamline treatment planning.
“AdvantageSim allows access to those technologies across the fleet to enable easy radiation, oncology, simulation, and planning,” said Newton.
FUJIFILM Medical Systems USA, Inc. highlighted the FCT Embrace CT system. Powered by Analogic, the FCT Embrace was the first 85-cm wide-bore CT imaging unit with 64- or 128-slice configurations. Optimized for oncology and radiology applications, the FCT Embrace, combined with other oncology solutions, offers CT simulation with radiation therapy treatment planning capabilities.
The company has enhanced the platform with the addition of FCT PixelShine (may not be commercially available in all countries), a novel deep-learning technique that improves the image quality of low-dose CT images, reducing the side effects of increased quantum mottle and image noise.
RaySearch Laboratories AB presented advances in machine learning and support for brachytherapy with online demos of its latest oncology software, including its treatment planning system RayStation and oncology information system RayCare. (Both products are subject to regulatory clearance in some markets.)
Among highlights in RayStation treatment planning system are support for brachytherapy planning and robust proton planning using machine learning. The upcoming release of RayStation 10B is expected in December 2020 (subject to regulatory clearance in some markets) and will add support for brachytherapy planning. It will feature automatic channel reconstruction in combination with dwell-time optimization to make the creation of high-quality brachytherapy plans faster and more consistent. Attendees experienced demos of machine-learning planning for photons and robust proton planning, as well as deep-learning organ segmentation.
“For machine learning, the big news is the support for robust proton planning. RayStation 10B now has the functionality to automatically generate proton plans by combining the machine-learning-based dose prediction with the robust optimization framework in RayStation. This [allows] for automatically generating both photon and proton plans that can be used for decision support,” said Fredrik Löfman, director of machine learning at RaySearch.
In the RayCare oncology information system, additional automation capabilities support scripting and enhanced workflow management tools. Released in June 2020, RayCare 4A promotes efficiency and safety by reducing routine manual tasks and handovers. Its workflow management capabilities are enhanced by the introduction of task management for clinical teams. Further functionalities in RayCare 4A include support for scripting as well as automated support for running RayStation scripts from tasks with forks – as well as several new patient chart features. RayCare 4B is expected in December 2020 (subject to regulatory clearance in some markets).
A therapeutic oncology company pioneering biology-guided radiation therapy (BgRT) for treating all stages of cancer, RefleXion Medical highlighted new research evaluating feasibility of BgRT to treat metastatic cancer. (The RefleXion X1 BgRT capability requires 510[k] clearance; this feature is not available for sale.)
RefleXion developed the first BgRT machine, which is cleared for the delivery of stereotactic body radiation therapy (SBRT), stereotactic radiosurgery (SRS) and intensity-modulated radiation therapy (IMRT). The first installation at Stanford University was completed in November, with plans to treat patients in early 2021.
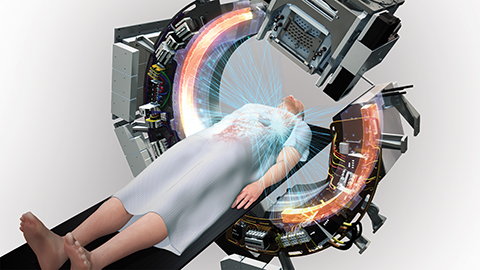
“The ring gantry system rotates 60 revolutions per minute. Because of that speed, it can deliver IMRT, SBRT and SRS with exceptional accuracy,” said Sean Shirvani, MD, senior vice president of Clinical and Medical Affairs at RefleXion. “On top of that architecture, we are adding a new functionality called BgRT, which uses [positron emission tomography] PET emission signals emanating from tumors to direct therapeutic radiation.” (Figure 2)
The BgRT technology will synchronize PET data with the linear accelerator to direct radiation therapy to tumors with sub-second latency.
RefleXion also entered into strategic collaborations in 2020 with Merck and Telix Pharmaceuticals, among others. “Our goal is to run clinical trials in collaboration with Merck that combine our technology with their immunotherapy agents to potentially enhance anti-tumor immunity,” said Dr. Shirvani. “As we look forward to incorporating other PET tracers besides FDG on our device, Telix will be at the forefront of those explorations with their cancer-cell-specific tracers for renal cell and prostate cancer.”
To address some of the most common challenges in radiation therapy, Siemens Healthineers AG featured two new CT simulators, AI technologies, and MR- and PET-based planning solutions.
Somatom go.Sim and Somatom go.Open Pro both feature an 85-cm bore and a 60-cm true scan field of view. To meet the challenge of precise 4D CT imaging, Somatom.go Open Pro features Direct i4D(1), the first 4D CT sequence that intelligently adapts to the patient’s breathing.
The MAGNETOM RT Pro includes optimized RT imaging protocols with features that allow the patient to be imaged in the treatment position. Dedicated quality assurance (QA) recommendations ensure consistent and geometric accurate MRI imaging. The system can provide important imaging applications to support accurate RT planning, including diffusion-weighted imaging to visualize tumor activity, MR-only planning with synthetic CT, and 4D MRI imaging to manage patient motion.
The company also showcased its Biograph mCT Sim PET/CT scanner, which features a large bore and gives clinicians access to intelligent AI-powered imaging applications to standardize protocols, personalize treatment, and perform respiratory motion correction for PET imaging. Earlier this year, Siemens announced a merger with Varian to create a multidisciplinary global health care company with a comprehensive cancer care portfolio. The transaction is expected to close in the first half of calendar year 2021.
“With Varian, we have the most comprehensive portfolio and multidisciplinary expertise in the industry to address the entire continuum of cancer care,” said Bernd Montag, CEO of Siemens Healthineers. “We want to help reduce the fear of cancer by ensuring that patients get the best chance right from the start.”
Belgium-based IBA (Ion Beam Applications S.A.) discussed how its Proteus solutions and network of clinical and industrial partners are shaping the future of proton therapy.
The latest developments for its Proteus systems address the current and future needs of proton therapy centers. IBA’s comprehensive motion management package will help clinicians treat more indications. IBA’s proton arc therapy will enable clinicians to treat patients precisely, faster and more easily. IBA systems are also capable of delivering FLASH proton therapy, which the company believes can radically change the radiation therapy landscape, revolutionizing cancer care in general. (Arc Therapy is a work in progress and FLASH therapy is in the research stage; neither are available for sale.)
“We believe that proton therapy systems need to be ready to manage motion and deliver ARC and FLASH therapy as soon as clinically released,” said Aymeric Harmant, global marketing director of proton therapy at IBA, noting that the company recently reached the milestone of treating 100,000 patients on IBA proton therapy systems.
With its open-vendor platform, IBA also integrates solutions of leading industrial partners to improve clinical workflow, integration and user experience. IBA has a long-term collaboration with Philips in imaging, diagnostics and radiation therapy for comprehensive cancer treatment. Additionally, its collaboration with Elekta is allowing both companies to offer a full set of treatment modalities in radiation therapy. Close collaboration with RaySearch Laboratories has demonstrated a fully integrated proton therapy workflow integrating IBA’s Proteus solution with advanced treatment planning system (TPS) RayStation and next-generation oncology information system (OIS) RayCare (subject to regulatory clearance in some markets).
Philips presented its dedicated radiation oncology line-up, including the Big Bore RT CT scanner and simulator for radiation oncology and therapy, and simulation platform and Ingenia MR-RT, the comprehensive MR simulation platform. Big Bore RT uses iterative model reconstruction (IMR) technology to help visualize fine detail and improve clinical accuracy for detecting and delineating small, subtle structures.
“One of the biggest things we hear from clinicians is that their image quality with our IMR feature has really improved. This is really helpful for better contouring, and also for better image quality in general,” said Ardie Ermers, vice president and general manager of Radiation Oncology at Philips.
The highlight of this year’s ASTRO meeting was the IntelliSpace Radiation Oncology workflow platform (Figure 3) (not available for sale in all markets), which enables radiation therapy departments to accelerate the time from patient referral to the start of treatment. The South West Wales Cancer Centre (SWWCC) in the UK believes it will help reduce its 14-step breast pathway referral-to-first treatment from 32 days to 14 days.
“The referral time to the radiation therapy department to start treatment is, on average, 15 to 20 days. With IntelliSpace, some of our customers are improving that workflow by 50%,” said Ermers.
Philips also continues to advance its leading MR-only radiation therapy portfolio with its AI-based MRCAT Brain application. MRCAT reduces the organization and coordination of scans involved in MR-CT registration, saving the patient from undergoing another imaging procedure.
“We used AI to speed up how the algorithm trains itself and to improve the quality of the MR-only simulation process,” Ermers said. “We know that AI for the sake of AI doesn’t really do much. So we make sure our AI can either make the workflow easier, more automated, or more streamlined, and remove the tedious manual tasks. We believe that applying AI should make life easier for users.”
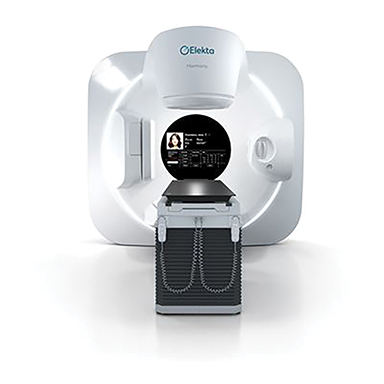
To help address the worldwide cancer burden, Elekta introduced the Elekta Harmony linear accelerator, (Figure 4) a cancer treatment system designed to meet the need for a productive, precise and versatile radiation therapy treatment system. Harmony recently received a CE mark, clearing the technology for commercial sales in Europe. (Elekta Harmony is not available in all markets.)
Harmony enables clinicians to treat most indications, including breast, lung, pelvic and head-and-neck cancers. With its comprehensive capabilities – combined with a shorter treatment slot of up to 25 percent, and a 30 percent smaller footprint than Elekta’s other linacs – Harmony is a practical system for developing and well-established markets.
“Elekta pioneered linear accelerators and we’re taking that technology to the next level, both in precision and personalization,” said Ioannis Panagiotelis, chief marketing and sales officer and executive vice president of Elekta. “We believe that real-time, high-quality diagnostic imaging is the way to go forward, and I feel very confident about the promise that this technology is bringing to the radiation oncology community.”
Elekta has enhanced its MOSAIQ radiation oncology portfolio with MOSAIQ Voice, a voice-enabled documentation and automation platform that uses speech recognition engines to reduce the time to create accurate patient notes. “Data demonstrate that by using MOSAIQ Voice, clinicians have saved on average 125 hours every year,” said Panagiotelis.
Elekta also acquired Kaiku Health, a cloud-based solution that monitors patient-reported outcomes, providing intelligent symptom tracking and management for health care providers in routine oncology care and studies. “Measuring what matters to patients is important in value-based health care, and we can demonstrate that we can improve the quality of life and also reduce potential additional costs.”
Innovations in hardware and software solutions and clinical data continue to support the use of Accuray Incorporated’s CyberKnife and TomoTherapy platforms, including the next-generation Radixact System, to deliver (ultra) hypofractionated radiation treatments. Hypofractionated radiation therapy — a shorter course of radiation therapy with higher radiation doses per fraction — provides an efficient and effective treatment option for an increasing number of indications.
With more than a decade of clinical proof behind Synchrony motion synchronization and real-time adaptive radiation therapy technology for the CyberKnife System, Accuray brought this advanced capability to the Radixact System. The AI-driven Synchrony technology corrects for tumors that move as a result of bodily processes, including respiration and digestion, as well as patient movement, without uncomfortable patient restraints, breath-hold techniques, or human intervention. It’s the only technology to use image guidance during radiation delivery to automatically adapt and synchronize radiation treatment in real-time with the movement of the tumor.
“With Synchrony, we can deliver dose to the target very efficiently because we follow it throughout its full cycle of motion, then we’re able to create tight margins around the tumor itself. So there’s no tradeoff between treatment delivery speed and quality,” said Corey Lawson, vice president of product strategy at Accuray.
The CyberKnife S7 System is a robotic, noninvasive radiation therapy device capable of treating cancerous and benign tumors throughout the body, as well as neurological disorders. The system supports SRS and SBRT techniques used to deliver (ultra) hypofractionated radiation therapy. New data presented at ASTRO showed that SBRT delivered with CyberKnife provides excellent control rates in men with early stage prostate cancer followed for at least 10 years. Treatment was also well-tolerated and efficacious in patients with early stage breast cancer.
The most recent innovation for the Radixact system, ClearRT Helical kVCT Imaging, is intended to quickly and cost-effectively produce clear, high-fidelity kVCT images that enhance soft-tissue visualization. (ClearRT helical kVCT imaging for the Radixact treatment delivery system is 510[k] pending and is not available for sale in any market.) The Radixact system is the only radiation therapy device with helical imaging, helical delivery, and intrafraction motion synchronization functionality using Synchrony, with the longest continuous imaging and treatment fields in the industry, up to 135 cm. “We can acquire very long field lengths very quickly, upwards of a meter in a minute,” said Lawson.
Citation
M B. Equipment Launches and Updates from ASTRO 2020. Appl Radiat Oncol. 2020;(4):40-44.
December 24, 2020