Diffusion-weighted imaging of the brain for glioblastoma: Implications for radiation oncology
Images
Magnetic resonance imaging (MRI) has become the foundation of diagnosis and monitoring in glioblastoma, and advanced techniques in data acquisition and analysis hold promise in improving clinical practice. Diffusion-weighted imaging (DWI) and diffusion-tensor imaging (DTI) represent 2 advanced image acquisition sequences that have been under development for over 2 decades and are now assuming a role in routine management of oncologic patients. These approaches are being investigated across body systems and have already established value in the diagnosis and management of prostate cancer.1-3 In a similar fashion in glioblastoma, the use of DWI and DTI has begun to expand outside of research settings and into patient care.
Rationale for diffusion imaging in neuro-oncology
This review focuses on diffusion imaging in glioma and, more specifically, on the ways DWI and DTI are becoming part of the management of patients with glioblastoma. These advances are based on the observation that tumor cell density is typically higher than normal tissue, and this increased cellularity leads to restricted extracellular water diffusion. DWI is sensitive to the degree of this water restriction and DTI offers a means of further quantifying the orientation of the restriction. The ability to use diffusion imaging as a noninvasive measure of cellularity has been demonstrated through extensive preclinical work. Recent studies have demonstrated that these methods might be used in patients to aid in the diagnosis and grading of tumors, estimate the extent of tumor infiltration, evaluate for residual or recurrent disease after an intervention, stratify a cohort based on the likelihood for an individual’s response, modify radiation contours to avoid low-risk areas and target areas with subclinical disease, improve neurosurgical approaches, look for early responses or progression, and differentiate between true progression and pseudoprogression. How to sort through these lofty goals and identify what holds the most promise will be part of the challenge in glioblastoma management. This review is aimed at helping the reader gain an understanding of the concepts of diffusion imaging and several of the emerging applications of the technique for radiation therapy utilization.
Principles of diffusion imaging
The effect of molecular diffusion on the magnetic resonance signal was noted in the classic paper on spin echoes by E.L. Hahn in 1950, which forms the basis of DWI and DTI to this day.4 Medical imaging is almost exclusively devoted to protons, which comprise about 2/3 of the atoms in the human body. Most of that is water, although the protons in the CH2 groups in fat can also be imaged. Water molecules will diffuse according to the principle of Brownian motion (i.e., random walk) unless restricted by their environment. If water molecules diffuse in any volume where the external magnetic field is inhomogeneous (e.g., through application of transient external field gradients), this will affect resonant echoes of the protons being probed by MRI. Within the body, cell membranes provide a barrier to diffusion. Fluid in the extracellular space will diffuse less readily in a crowded environment, such as in cases of tumor infiltration or when cells swell from a shift of fluid intracellularly where restriction is high. Diffusion MRI pulse sequences allow detection of both the magnitude and direction of water diffusion.
Diffusion-weighted image acquisition
The concept of how diffusion of water molecules can affect their resonant signals is easy to grasp, but the physics and mathematics for this procedure are extremely technical in detail. Interested readers are referred to a very concise review by Le Bihan et al., or to Koh and Collins for an excellent overview of general clinical applications.5,6
For the purposes of this review, we must introduce some commonly used mathematical terms. In the idealized case of isotropic diffusion, and ignoring other flows such blood, the DWI signal would be expected to have a simple exponential relationship where b is an experimentally adjustable parameter that includes the timing and magnitude of the external magnetic field gradients used in the DWI pulse sequences, D is the diffusion coefficient, and A0 is the signal in the absence of diffusion:
A = A0 exp(-bD)
For water in an isotropic medium, D = 2 x 10-3 mm2/s at room temperature; note that the viscosity of water and, hence, self-diffusion is very temperature-dependent, with an increase of about 2% per degree C. Higher b values essentially mean that the image was acquired allowing longer times for diffusion to affect the signals detected. With b=0, one gets a standard T2-weighted image that depends on the other acquisition parameters.
By acquiring DWI images at even a few b-values, one can estimate the diffusion coefficient for the resonant protons. In fact, this is an empirical parameter since it likely includes other flow mechanisms besides simple Brownian motion. The result is called the ADC, the apparent diffusion coefficient. As the review by Koh and Collins notes, ADC’s are often reported as indices of therapeutic response, but the actual numbers obtained for ADC can vary widely depending on the exact details under which the DWI images were taken, and standardization is lacking.6
Diffusion-tensor imaging
In the cerebrospinal fluid, diffusion is largely unrestricted and the motion of water molecules is isotropic. In white matter tracts, diffusion is restricted by axonal cell membranes and water diffuses anisotropically. By quantifying diffusion anisotropy along multiple directions within a tissue, the density and orientation of the cellular structure within a unit of measurement (i.e., a voxel) in an MRI image can be estimated.
A diffusion tensor is the mathematical description of the three-dimensional orientation of an ellipse, which in DTI represents the principal direction of water motion. While DWI acquires data in only 3 directional planes, DTI requires that at least 6 directions be imaged in order to calculate a diffusion tensor. The greater the number of diffusion directions acquired, the more accurately the calculated diffusion tensor represents the true direction of water movement.
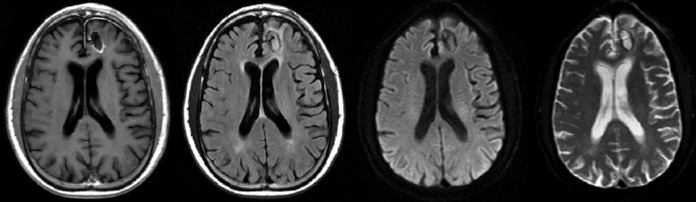
Common parameters derived from DTI include the fractional anisotropy (FA) and mean diffusivity (MD). Occasionally, the components of fractional anisotropy are analyzed independently, such as axial and radial diffusivity or the isotropic and anisotropic components of FA. MD is used interchangeably with ADC, since the ADC is an average of the measured diffusion directions. While ADC and MD represent the magnitude of diffusion, FA describes the degree of anisotropy of diffusion. For example, in the body of the corpus callosum, diffusion is anisotropically oriented along the axis of fiber tracts as they cross from one hemisphere to another. The magnitude of FA will decrease as the anisotropy decreases, such as in areas of crossing fibers with different orientations or where there is more gray matter. FA, which varies between 0 and 1, will be near-0 in cerebrospinal fluid (CSF) and near-1 in white matter. An example demonstrating qualitative differences of MRI sequences in glioblastoma is shown in Figure 1.
Because DTI quantifies the degree of anisotropy across voxels in the brain based on the orientation of fiber tracts, a voxel-wise analysis may be performed to generate white matter fiber tracts. DTI tractography has developed into a large field of study with broad research and potential clinical applications.
Standard clinical applications
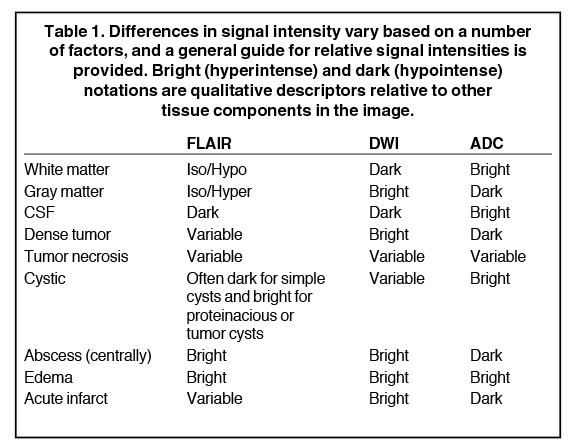
Clinicians are most familiar with diffusion imaging in the setting of neurovascular injury. DWI began being applied in the setting of stroke evaluation approximately 2 decades ago and is now routinely used in clinical practice to determine the presence and chronicity of stroke evolution.7,8 In the setting of acute ischemia, it is hypothesized that extracellular fluid moves into the intracellular compartment where diffusion is relatively restricted. This displays a pattern of restriction, where areas of acute ischemia appear hyperintense (i.e., high value) on DWI and hypointense (i.e., low value) on ADC images. As ischemia resolves over the course of several weeks, ADC increases above normal and serves to highlight areas of more chronic injury. The sensitivity of DWI and ADC to distinguish between acute and chronic injury has led to their application as the standard imaging sequences in evaluating stroke. Other clinical uses of diffusion imaging include evaluating infection (e.g., abscesses), inflammation, demyelination (e.g., multiple sclerosis), edema, cysts and trauma. Signal intensities for benign and pathologic imaging findings vary across MRI sequences and a general overview of common descriptors is provided in Table 1.
Experimental applications in glioblastoma
Improved diagnosis and histologic subclassification
MRI provides a noninvasive means of identifying intracranial pathologies, and diffusion imaging has a natural role in separating certain benign (e.g., cystic or infectious) lesions from malignancies. There has been considerable interest in diagnostic radiology to apply diffusion imaging techniques in the setting of suspected neoplasia within the central nervous system. Innumerable studies in the literature aim to correlate diffusion parameters across disease sites with various pathologic findings and patient outcomes. These studies hypothesize that tumors with high cellular density will restrict extracellular diffusion and correlate with diffusion parameters.
An important topic in this area is the diagnosis and grading of primary glial tumors through imaging. A reliable noninvasive diagnostic method for suspected glioma may be useful in cases where a brain biopsy either cannot be safely obtained or if a biopsy is attempted and is nondiagnostic. Perhaps a more frequently encountered scenario is when a biopsy is positive for a low-grade glioma, but the presence of new clinical symptoms or the appearance of the disease by standard radiographic techniques suggests a more aggressive histology. In these cases, biopsy results indicating low-grade histology may be due to random sampling, such as in a lesion with mixed features. Furthermore, it may be possible to follow patients with a low-grade glioma to noninvasively monitor for conversion to a higher grade.
Preclinical work in rodent models has motivated the application of diffusion imaging to estimate tumor cellularity in humans. These studies have been discussed elsewhere and demonstrate a high sensitivity of diffusion parameters to changes in the cellular density of a tumor.9-11 However, there are many added limitations when applying DWI in humans (e.g., patient heterogeneity, lower field magnets, head motion, restricted scan time and data acquisition, etc.). To test the ability of diffusion imaging to quantify the cellular density in humans, Sugahara and coworkers acquired DWI data from 20 patients with pathologically confirmed glial cell tumors.12 The minimum ADC value was correlated with the density of cellular nuclei across a standard pathologic slide, and the authors found a strong relationship (r = -0.77) between the 2 variables. In a similar study of 10 patients, including WHO grade II and IV tumors, the authors also found a relationship between ADC and cell density of the same magnitude (r = -0.77).13 In one of the largest series to examine this question, a Chinese group retrospectively evaluated over 100 glial tumors and correlated the minimum ADC value with glioma grade.14 In their study, minimum ADC was significantly correlated with grades 2, 3 and 4 glioma (r = -0.524) and a weaker, but statistically significant, relationship was found between the minimum ADC and the Ki-67 mitotic labeling index (r = -0.312). These data along with other studies help to confirm preclinical findings that highly cellular tumors are associated with low ADC values, which supports this modality as a noninvasive measure of tumor grade.15,16
The tendency of glioblastoma to infiltrate along white matter tracts often leads to extensive disease and may underlie multifocal presentations or recurrences after treatment. Currently, edema (i.e., radiographically T2/FLAIR positive) is the best marker for the subclinical spread of tumor, but is a qualitative measure and is not specific to changes due to tumor infiltration. Diffusion imaging, particularly DTI, may offer an improved means of evaluating the presence of subclinical disease that is not only qualitative, but may also quantify spatial and temporal changes to assist in radiation treatment planning.
Because white matter is arranged in a highly organized pattern, diffusion is similarly restricted along the axis of fiber tracts, but as glioma cells infiltrate these tracts, they disrupt this order along with anisotropic diffusion (i.e., fractional anisotropy [FA]). Therefore, DTI measures should be sensitive to infiltrating the tumor and be particularly useful in identifying glioblastoma. This idea has been illustrated in a comparison between high-grade glioma and other brain tumors.17 However, while some studies of FA have been promising, 18-20 others have questioned the utility of FA over other radiographic measures, including other diffusion parameters.21-23 Many of these studies suffer from small sample sizes and a heterogeneous population of patients making negative studies difficult to interpret.
The most definitive test for FA in estimating the presence of a tumor is through pre-biopsy measurements. In one of the few studies to address this, 19 patients were scanned with DTI prior to stereotactic biopsy of brain tissue.24 Here, FA was found to significantly correlate with cell density (r=0.73) and the Ki-67 labeling index (r = 0.8). Similarly, a Japanese group utilized DTI to calculate the FA and MD values within pathology-confirmed gliomas of grades I – IV.20 In 41 patients, the authors found a clear separation in FA values between high grade (III – IV) and low grade (I – II) glioma. MD served less well as a marker for tumor grade, but results were still in-line with previous reports showing increased values for grade I relative to higher grade tumors. These studies represent a small sample of the promising work being done to apply DTI in clinical settings, but there are limitations in image quality, lengthened acquisition time and labor-intensive data analysis to overcome before the methods are more widely adopted.
Predicting and evaluating treatment response for glioblastoma
DWI has been proposed as a potential marker of early treatment response and may be used to predict who will benefit from a particular therapy before initiation of radiation treatment. The rationale is that necrotic tumors and/or those low in vascularity may be more resistant to either systemic agents (e.g., due to decreased delivery) or radiation (e.g., due to poor tumor oxygenation). Additionally, if a tumor is sensitive to treatment, its cellularity is expected to decrease either during or soon after a course of therapy. The role of diffusion imaging in respect to this topic has been investigated and reviewed largely by a group at the University of Michigan, who has performed a number of seminal studies on the topic.25 They have reported results from a cohort of 60 patients with WHO grade III or IV glioma.26 In this prospective study, DWI data were acquired at 3 time points during the course of treatment. Rather than taking an intra-lesion measure of ADC, a voxel-wise analysis was performed on each brain, and changes in this map across time were correlated with survival outcomes. Intriguingly, the authors found that increases in diffusion at 3 weeks into a course of radiotherapy were associated with improved rates of survival at 1 year. Other work has supported this use of diffusion imaging in guiding conventional radiotherapy and stereotactic radiosurgery with promising results.27-32
Bevacizumab is one systemic agent that has been used to treat glioblastoma, although despite promising phase II data it was not shown to improve overall survival in a randomized controlled trial.33 However, there is interest in identifying a subgroup of glioblastoma patients for whom bevacizumab may be appropriate, particularly because the drug improved progression-free survival when added to standard management. Ideally, a pretreatment marker would be used to predict response to the intervention, and this could be used to stratify patients in future trials. A group at the University of California, Los Angeles has proposed a method for filtering the ADC histogram taken from non-necrotic portions of tumor. These values likely correlate with more densely cellular regions of tumor, and the authors found that they were a reliable biomarker to predict a response to bevacizumab.34 Moreover, the authors found that their quantification of the ADC distribution was a superior predictor of progression-free survival relative to the Macdonald criteria, a standardized post-treatment radiographic assessment.35 Others have begun to validate the ability of ADC to serve as a biomarker of response and have published encouraging results.36,37
ADC may also serve as a useful biomarker for early responsiveness to the current standard of care. The success of temozolomide (TMZ) and radiation therapy in a large randomized trial has established it as the first-line chemotherapeutic agent in this disease.38,39 Furthermore, important work in understanding the biochemical mechanism of its effectiveness has led to a valuable epigenetic marker for predicting treatment response.40 These advances have changed management of glioblastoma and revealed further subgroups of patients who may experience relatively dramatic improvements in survival. How to identify these patients early in their course of therapy has become an important question, particularly with the hope that novel interventions will provide options for alternative treatment courses for poor-responders. To test the ability of diffusion imaging to predict response to TMZ, Khayal and coworkers evaluated MRI data before, during and immediately after a course of concurrent RT-TMZ for glioblastoma.41 The authors acquired DWI and DTI data between 3-5 weeks into a course of radiotherapy with daily TMZ. They quantified several diffusion parameters at this time point and compared these to a post-treatment scan. Here, an increase in ADC after chemoradiation relative to during treatment was correlated with a lower risk of 6-month progression. Many studies are underway to further characterize the ability of various diffusion measures to predict and evaluate treatment responses. Published literature has begun to establish this role, but should be interpreted with caution as these methods are experimental, often involve a small number of patients, and are subject to publication bias. Another significant problem raised on Koh and Collins review was the lack of standardization in the DWI acquisition parameters, which can affect results.6 With the work of the Michigan group as a foundation, clinical trials may soon test the utility of DWI to assess a patient’s disease during treatment. A convincing study will ensure standardized data acquisition and analysis techniques with quality assurance measures to remove poor-quality MRI scans. The implication is that DWI may provide an early marker of treatment response to guide modifications in radiation target delineation and dose prescription as well as chemotherapeutic modifications.
Monitoring for recurrence
In addition to predicting or tracking response to treatment, there is an interest in utilizing DWI to estimate the probability of recurrent disease after radiation therapy. Noninvasively assessing the existence of recurrent disease has long been a challenge due to post-surgical and radiation changes along with pseudoprogression or pseudoresponse.42,43 This is a commonly encountered problem, with up to 50% of patients treated with standard management found to have pseudoprogression, with each case causing a clinical dilemma in determining further management.44 Standard radiographic methods have been proposed, such as the Macdonald, RECIST and RANO criteria, but additional measures are clearly needed.35,45-47 As described above, diffusion imaging is a sensitive measure of tissue cellularity, but its susceptibility to post-treatment artifacts has not been clearly defined.
To compare the ability of ADC values to distinguish progression versus treatment-induced changes, one study analyzed data from 18 patients in whom 7 had histologic confirmation of tumor recurrence.48 ADC values were significantly lower on tissue identified as recurrent disease relative to areas of non-recurrence. These data are consistent with other work that has relied on long-term radiographic follow-up to distinguish progression versus pseudoprogression, but additional studies that utilize post-treatment biopsy or resection tissue are needed.49-51
Other work is underway to develop a multimodality approach, as opposed to a single measure, to describe post-treatment changes. In a recent example, Cha et al., analyzed patients who received standard therapy with surgery plus adjuvant chemoradiotherapy and were found to have possible radiographic progression.52 Both ADC and regional cerebral blood flow (rCBV) histograms were generated from enhancing areas that were questionable for progression versus pseudoprogression. The authors found that the combined analysis was superior to either analysis alone, and that the multiparametric measure was predictive of progression-free and overall survival. They hypothesize that ADC and rCBV are particularly well-suited in evaluating tumor recurrence because they each supplement for some of the deficiencies of the other. This principle has been applied in several other investigations aimed at developing multimodality measures (see below).
Overcoming limitations and future directions
Special challenges in data acquisition
A major limitation of single measures of ADC (e.g., minimum, maximum or mean) is the information lost in data reduction. This may greatly reduce the sensitivity of ADC measures of glioma, which are often heterogeneous lesions with areas of dense tumor, necrosis, cystic fluid, and hemorrhage all potentially within a single focus of disease. Many of the studies mentioned in this review are limited in this regard and others have described more sophisticated approaches to data analysis.
One method to address this obstacle is to perform voxel-wise ADC analyses as proposed by Moffat and colleagues,30 or to perform ADC histogram analyses as described in several of the studies reviewed here.34,52 This may be a particularly important consideration when analyzing tumor responses to therapy because there may be a nonuniform response to treatment that may not be captured by taking, for example, the mean ADC value across the full volume of a lesion, or even the non-necrotic portion of a tumor.
Another limitation is the variability in DWI sequences from one center to another, between scanners in the same center or in a single scanner across time. Also, the reliability of ADC depends on how the data are acquired. For example, diffusion neuroimaging in most practices is carried out by acquiring a b0 (i.e., T2-weighted) image and a b1000 image. ADC is calculated from these 2 points, which places the accuracy of the measure upon the quality of only 2 images. Furthermore, higher b-values are impaired by an inherently low signal-to-noise ratio. Added to the natural susceptibility of echo-planar imaging to artifacts and the particular sensitivity of diffusion-weighted imaging to head motion, these factors make high-quality data acquisition a challenge.53,54 One way to improve data quality is through scan averaging, where multiple diffusion sequences are acquired and merged post-hoc. This can improve the accuracy of ADC while maintaining image resolution, particularly when data are acquired at higher field strengths. Another approach to improving accuracy is through acquiring additional b-values, which is more often performed in diffusion imaging of other body systems. The difficulties in obtaining high-quality diffusion data make it difficult to interpret negative studies, particularly when performed on a small cohort. Furthermore, in the clinical setting there are practical limitations to scan time, and these added MRI sequences must be well-justified both from the perspective of scan time for patients and health care costs.
Other challenges relate to data analysis, which can be time- and labor-intensive, and require proper equipment and expertise. While many commercial and public software packages are available, developing more standard protocols for data processing and quality assurance must precede adopting these techniques to general clinical practice.
Determining radiation treatment volumes
Over the past several decades, radiation treatment volumes for glioma have decreased from whole-brain irradiation with two-dimensional planning to three-dimensional conformal partial brain radiotherapy often utilizing intensity-modulated radiation.33,55,56 It has also been well-established that the majority of tumor recurrence occurs within the high-dose treatment volume, and that boosting presumed high-risk areas does not necessarily improve local control.57-59 These findings bring into question the current standard in treatment volume delineation. While lower overall doses may negatively impact survival, it may be possible to continue the trend toward smaller treatment volumes by using diffusion imaging to tailor the high-dose region to areas at highest risk for subclinical spread.60 Rather than using a somewhat arbitrary, but still standard 2 cm margin upon T2/FLAIR hyperintensity, a more sophisticated method of estimating subclinical spread of disease is needed. It may be that dose escalation studies have failed to improve outcomes because of overtreatment of low-risk areas of viable brain without sufficient dose intensification to the highest risk regions.
Many groups deviate from the traditional treatment volumes used in RTOG studies, but evidence-based alternatives to target delineation is lacking. Investigations by Price and colleagues have begun to address this.17,19,61 In one study, the authors performed a prospective analysis of 20 patients who underwent standard and diffusion-tensor MRI followed by stereotactic or image-guided biopsies.61 Histopathologic findings were correlated with the voxels corresponding to the sampled area and DTI data were analyzed. The authors found that the degree of isotropic and anisotropic diffusion was closely correlated with the presence of gross and infiltrative tumor. In fact, they describe their method as being 98% sensitive and 81% specific for disease.
Data-driven methods for defining radiation treatment volumes are unlikely to improve local control, but will likely reduce exposure of viable brain to high doses of radiation. With modest, but important improvements in survival with modern care, brain re-irradiation is becoming more common for treatment glioblastoma and is being tested in an RTOG trial (http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1205). By sparing as much normal tissue as possible in a patient’s initial course of radiotherapy, additional radiation in the recurrent setting might fall within an acceptable therapeutic window.
Neurosurgical planning
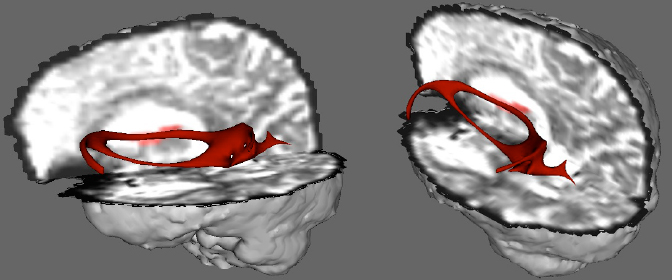
Somewhat more experimental is the application of DTI tractography for neurosurgical planning purposes. Functional neuroimaging is used in some centers to better identify eloquent brain regions, such as language, motor or sensory cortices in an effort to preserve function after tumor resection.62-65 DTI may improve delineation of important white matter fiber tracts that may be displaced or deformed due to tumor mass effects. Figure 2 illustrates a patient with a large tumor in the left temporal lobe that was planned for surgical resection. DTI and fiber tractography were performed between the left inferior frontal gyrus representing Broca’s area and the left superior temporal gyrus representing Wernicke’s area. Robust fibers were identified representing the arcuate fasciculus and mass effect from the tumor not only displaced the fibers medially, but also appeared to separate the arcuate into a superior and inferior tract with no coherent fiber orientation between the two. In conjunction with other presurgical and intraoperative assessments, these images can guide the resection approach and extent. Early work in this area has demonstrated the feasibility of the method with neurosurgical and even radiosurgical planning, but future studies are needed to determine its role in improving resection and treatment outcomes.66,67
Multifactorial modeling
The evidence presented in this review demonstrates a significant role for diffusion MRI techniques for the non-invasive clinical management of brain tumor patients. Along with improvements in diffusion MRI acquisition and analysis, there have been concurrent gains in other noninvasive strategies of tumor assessment. Namely, PET, MR spectroscopy and cerebral blood flow measures have become available in clinical practice and may be used in combination with diffusion imaging to help guide treatment decisions.
Numerous studies have begun to investigate the ability of multimodality imaging to better characterize tumors. As mentioned above, a recent study described a method for combined rCBV and ADC to distinguish between progression and pseudoprogression.52 The same group has applied the method to evaluating brain metastases.68 Others have employed combinations of imaging modalities to improve diagnostic capabilities,69,70 perform noninvasive histologic assessment,71,72 distinguish between disease and radiation injury,73 and to correlate pre-treatment imaging with survival.74
A goal of research in this area is to formulate a standard multiparametric model to assess for the presence of subclinical disease. This would aid in nearly every stage of management for brain tumor patients, from diagnosis to tailoring treatment regimens. As these multiple noninvasive techniques continue to be refined, it will become more important for clinicians to understand their potential roles and limitations.
Summary
At present, diffusion imaging is not a primary modality in the diagnosis and characterization of glioblastoma, but practical applications are being developed. Results from prospective studies to validate findings from retrospective series have indicated how diffusion parameters may be correlated with tumor cellularity, invasiveness and prediction of treatment response. Early work has demonstrated the feasibility of altering radiation target volumes based on findings from diffusion imaging, but there have been no trials to date implementing this in treatment planning.
MRI continues to develop fascinating imaging views due to the ability to manipulate the nuclear spin resonance via precisely determined rf pulse sequences, and also the addition of pulsed magnetic field gradients of sufficient size to dwarf any inhomogeneities in the static field created by the main superconducting coils. Taking advantage of differences in the nuclear spin relaxation times of protons in different tissues, rf pulse sequences can contrast out some tissues (or make them the main detected signal), at the discretion of the MRI experimentalist. DWI and DTI open new windows for the clinician due to its potential to directly observe small regions of high cellularity, which may indicate otherwise unobservable areas of malignancy both before and after standard treatment.
The most promising role of diffusion imaging may be in multiparametric analyses where advances in several modalities may be considered together. The next step in applying these techniques to clinical practice will be formalizing a standard means of data acquisition, analysis and interpretation. Functional diffusion maps and multimodal ADC histogram models appear to be the most promising approaches at this time and will require further validation. Finally, clinical trials involving larger numbers of patients with strictly defined imaging protocols are needed to move the promising experimental results into generalized clinical disease management for glioma.
Acknowledgements: We would like to thank Dr. Timothy Marinetti, senior grants specialist, Department of Radiation Oncology, Columbia University Medical Center, for his extensive assistance in the preparation of this manuscript. We would also like to thank Dr. Krishna Surapaneni, assistant professor of Clinical Radiology, Temple University School of Medicine, Philadelphia, PA, and Dr. Bryan Lanzman, chief resident, Department of Radiology, New York Presbyterian Hospital—Columbia University Medical Center, for their insightful comments.
References
- Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: Recommendations from a European consensus meeting. Eur Urol. 2011;59:477-494.
- Hambrock T, Hoeks C, Hulsbergen-Van De Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012;61:177-184.
- Sciarra A, Barentsz J, Bjartell A, et al. Advances in magnetic resonance imaging: How they are changing the management of prostate cancer. Eur Urol. 2011;59:962-977.
- Hahn EL. Spin echoes. Physical Review. 1950;80:1-22.
- Le Bihan D, Turner R, Moonen CT, et al. Imaging of diffusion and microcirculation with gradient sensitization: Design, strategy, and significance. J Magn Reson Imaging. 1991;1:7-28.
- Koh DM, Collins DJ. Diffusion-weighted MRI in the body: Applications and challenges in oncology. AJR Am J Roentgenol. 2007:188;1622-1635.
- Lansberg MG, Thijs VN, O’Brien MW, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol. 2001;22:637-644.
- Lutsep HL, Albers GW, DeCrespigny A, et al. Clinical Utility of Diffusion-weighted Magnetic Resonance Imaging in the Assessment of Ischemic Stroke. Ann Neurol. 1997;41:574-580.
- Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: An early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029-2036.
- McConville P, Hambardzumyan D, Moody JB, et al. Magnetic resonance imaging determination of tumor grade and early response to temozolomide in a genetically engineered mouse model of glioma. Clin Cancer Res. 2007;13:2897-2904.
- Moffat BA, Hall DE, Stojanovska J, et al. Diffusion imaging for evaluation of tumor therapies in preclinical animal models. Magma. 2004;17: 249-259.
- Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9:53-60.
- Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001;22: 1081-1088.
- Chen Z, Ma L, Lou X, et al. Diagnostic value of minimum apparent diffusion coefficient values in prediction of neuroepithelial tumor grading. J Magn Reson Imaging. 2010;31:1331-1338.
- Higano S, Yun X, Kumabe T, et al. Malignant astrocytic tumors: Clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiol. 2006;241:839-846.
- Tozer DJ, Jager HR, Danchaivijitr N, et al. Apparent diffusion coefficient histograms may predict low-grade glioma subtype. NMR in Biomed. 2007;20:49-57.
- Price SJ, Burnet NG, Donovan T, et al. Diffusion tensor imaging of brain tumours at 3 T: A potential tool for assessing white matter tract invasion. Clin Radiol. 2003;58:455-462.
- Deng Z, Yan Y, Zhong D, et al. Quantitative analysis of glioma cell invasion by diffusion tensor imaging. J Clin Neurosci. 2010;17:1530-1536.
- Price SJ, Peña A, Burnet NG, et al. Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radio. 2004;14: 1909-1917.
- Inoue T, Ogasawara K, Beppu T, et al. Diffusion tensor imaging for preoperative evaluation of tumor grade in gliomas. Clin Neurol Neurosurg. 2005;107:174-180.
- Sinha S, Bastin ME, Whittle IR, et al. Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am J Neuroradiol. 2002;23:520-527.
- Tropine A, Vucurevic G, Delani P, et al. Contribution of diffusion tensor imaging to delineation of gliomas and glioblastomas. J Magn Reson Imaging. 2004;20:905-912.
- van Westen D, Latt J, Englund E, et al. Tumor extension in high-grade gliomas assessed with diffusion magnetic resonance imaging: values and lesion-to-brain ratios of apparent diffusion coefficient and fractional anisotropy. Acta Radiol. 2006;47: 311-319.
- Beppu T, Inoue T, Shibata Y, et al. Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg Neurol. 2005;63:56-61.
- Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007;25:4104-4109.
- Hamstra DA, Galbán CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: Correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26:3387-3394.
- Hamstra DA, Chenevert TL, Moffat BA, et al. Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci USA. 2005;102:16759-16764.
- Mardor Y, Pfeffer R, Spiegelmann R, et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-Value diffusion-weighted magnetic resonance imaging. J Clin Oncol. 2003;21:1094-1100.
- Mardor Y, Roth Y, Ochershvilli A, et al. Pretreatment prediction of brain tumors’ response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia. 2004;6:136-142.
- Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci USA. 2005;102:5524-5529.
- Saksena S, Jain R, Narang J, et al. Predicting survival in glioblastomas using diffusion tensor imaging metrics. J Magn Reson Imaging. 2010;32:788-795.
- Tomura N, Narita K, Izumi J, et al. Diffusion changes in a tumor and peritumoral tissue after stereotactic irradiation for brain tumors: possible prediction of treatment response. J Comput Assist Tomogr. 2006;30:496-500.
- Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;20:699-708.
- Pope WB, Kim HJ, Alger J, et al. Recurrent glioblastoma multiforme : ADC histogram analysis predicts response to bevacizumab treatment. Radiol. 2009;252:182-189.
- Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277-1280.
- Jain R, Scarpace LM, Ellika S, et al. Imaging response criteria for recurrent gliomas treated with bevacizumab: role of diffusion weighted imaging as an imaging biomarker. J Neurooncol. 2010;96:423-431.
- Jain R., Narang J, Sundgren PM, et al. Treatment induced necrosis versus recurrent/progressing brain tumor: going beyond the boundaries of conventional morphologic imaging. J Neurooncol. 2010;100:17-29.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459-466.
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003.
- Khayal IS, Polley MC, Jalbert L, et al. Evaluation of diffusion parameters as early biomarkers of disease progression in glioblastoma multiforme. Neuro Oncol. 2010;12:908-916.
- Brandsma D, Stalpers L, Taal W, et al.. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453-461.
- Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22: 633-638.
- Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405-410.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963-1972.
- Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10:361-367.
- Hein PA, Eskey CJ, Dunn JF, et al. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25:201-209.
- Chu HH, Choi SH, Ryoo I, et al. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiol. 2013;269:831-840.
- Lee WJ, Choi SH, Park C, et al. Diffusion-weighted MR Imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad Radiol. 2012;19:1353-1361.
- Smith JS, Cha S, Mayo MC, et al. Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J Neurosurg. 2005;103:428-438.
- Cha J, Kim ST, Kim HJ, et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol. 2014;35:1309-1317.
- Edelman RR, Wielopolski P, Schmitt F. Echo-planar MR imaging. Radiol. 1994;192:600-612.
- Jezzard P, Balaban RS. (1995). Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65-73.
- Salazar O M, Rubin P, Feldstein ML, et al. High dose radiation therapy in the treatment of malignant gliomas: final report. Int J Radiat Oncol Biol Phys. 1979;5:1733-1740.
- Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725-1731.
- Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635-1642.
- Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853-860.
- Cardinale R, Won M, Choucair A, et al. A phase II trial of accelerated radiotherapy using weekly stereotactic conformal boost for supratentorial glioblastoma multiforme: RTOG 0023. Int J Radiat Oncol Biol Phys. 2006;65:1422-1428.
- Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64:769-774.
- Price SJ, Jena R, Burnet NG, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: An image-guided biopsy study. AJNR Am J Neuroradiol. 2006;27:1969-1974.
- Hirsch J, Ruge MI, Kim KH, et al. An integrated functional magnetic resonance imaging procedure for preoperative mapping of cortical areas associated with tactile, motor, language, and visual functions. Neurosurg. 2000;47:711-722.
- Martino J, Honma SM, Findlay AM, et al. Resting functional connectivity in patients with brain tumors in eloquent areas. Ann Neurol. 2011;69:521-532.
- Tharin S, Golby A. Functional brain mapping and its applications to neurosurgery. Neurosurg. 2007;60:185-201.
- Vlieger E, Majoie CB, Leenstra S, et al. Functional magnetic resonance imaging for neurosurgical planning in neurooncology. Eur Radiol. 2004;14:1143-1153.
- Conti A, Pontoriero A, Ricciardi GK, et al. Integration of functional neuroimaging in CyberKnife radiosurgery: feasibility and dosimetric results. Neurosurg Focus. 2013;34:E5.
- González-Darder JM, González-López P, Talamantes F, et al. Multimodal navigation in the functional microsurgical resection of intrinsic brain tumors located in eloquent motor areas: role of tractography. Neurosurg Focus. 2010;28:E5.
- Cha J, Kim ST, Kim HJ, et al. Analysis of the layering pattern of the apparent diffusion coefficient (ADC) for differentiation of radiation necrosis from tumour progression. Eur Radiol. 2013;23:879-886.
- Bulakbasi N, Kocaoglu M, Ors F, et al. Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of common brain tumors. AJNR Am J Neuroradiol. 2003;24:225-233.
- Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678-687.
- Gupta RK, Cloughesy TF, Sinha U, et al. Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J Neurooncol. 2000;50:215-226.
- Yang D, Korogi Y, Sugahara T, et al. Cerebral gliomas: prospective comparison of multivoxel 2D chemical-shift imaging proton MR spectroscopy, echoplanar perfusion and diffusion-weighted MRI. Neuroradiol. 2002;44:656-666.
- Zeng Q, Li C, Liu H, et al. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2007;68:151-158.
- Oh J, Henry RG, Pirzkall A, et al. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging. 2004;19:546-554.