CB-CHOP: A simple acronym for evaluating a radiation treatment plan
Images
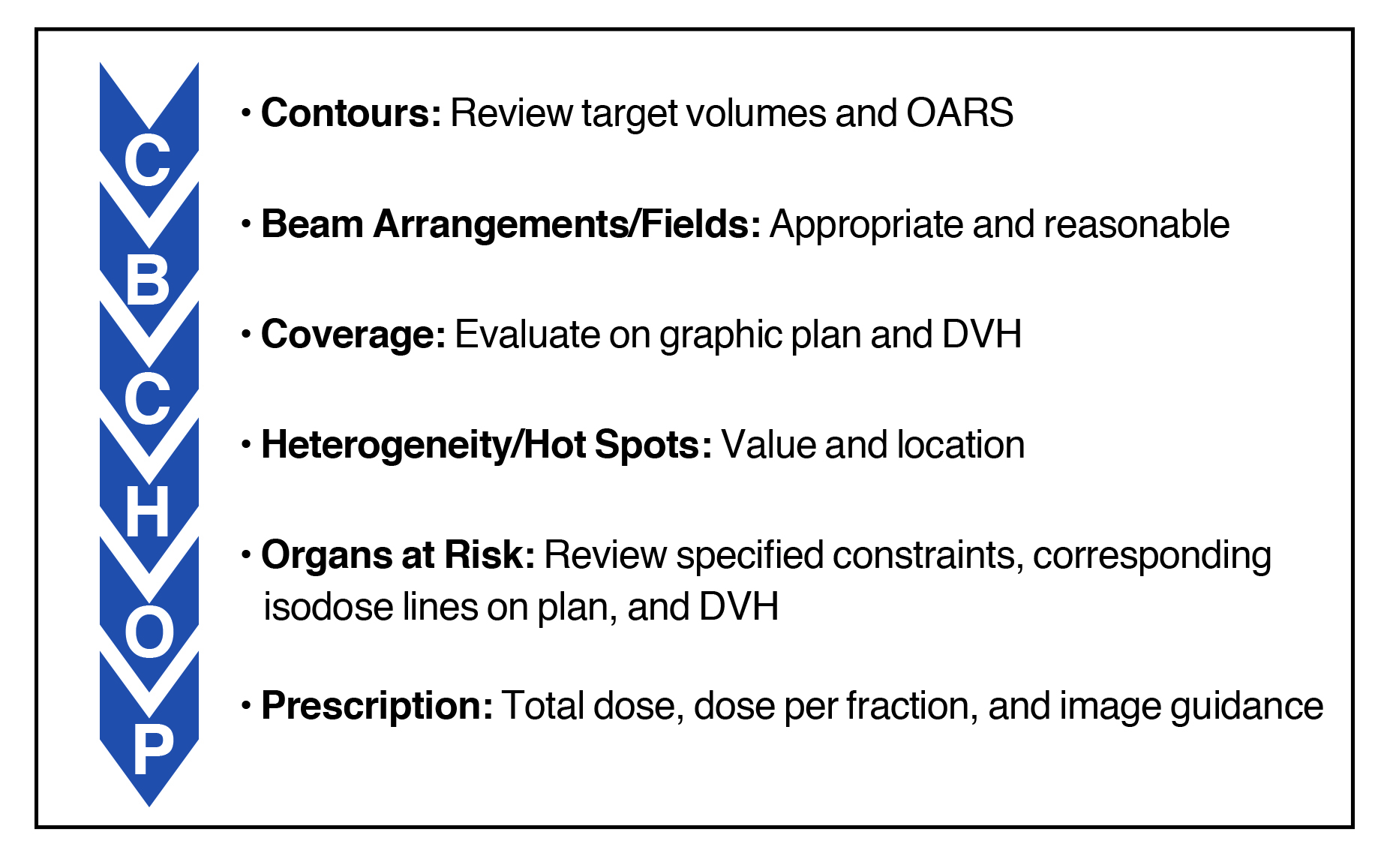
Evaluating a radiation plan is an essential task for the radiation oncologist that is becoming more complex due to advances in radiation techniques. Multiple components are required to ascertain the quality and acceptability of a radiation therapy plan, which can be difficult to remember for the radiation oncologist in training. Herein is proposed a systematic approach for plan evaluation to ensure all aspects are properly assessed prior to approval. First proposed by Dr. Raymond Mak at Brigham and Women’s Hospital/Dana-Farber Cancer Institute, the approach is described by the acronym CB-CHOP, which stands for contours, beams, coverage, heterogeneity, organs at risk, and prescription.
CB-CHOP Components
Contours
When a radiation plan has been generated for physician review, the radiation oncologist should first review the delineated target volumes and organs at risk (OAR) or normal structures. It is important to ensure that all appropriate OARs are accounted for and contoured accurately, especially when some OAR contouring is delegated to others. The reviewing radiation oncologist may find that a normal structure was forgotten and mistakenly not contoured, or that the isodose lines spill into an OAR initially thought not to be at risk and as such was not contoured. This step is also an opportunity to re-check the target volume contours and ensure that at-risk areas are delineated and provided dose in their entirety. Any expansions should be reviewed for accuracy. For example, a gross tumor volume (GTV) may have been modified without appropriate re-expansion of the corresponding clinical target volume (CTV) and planning target volume (PTV).
Beam Arrangements/Fields
The next step is to evaluate the radiation therapy (RT) field arrangement and delivery technique, which ranges from simple single or opposed fields to complex volumetric-modulated arc therapy (VMAT) plans. The delivery technique is typically specified by the physician prior to planning, and in modern practice the beam arrangements are left to the discretion of the dosimetrist. One should therefore take note of the beam arrangements used by the dosimetrist to generate the plan.
For 3-dimensional (3D) plans, it is important to ensure that the fields are entering the body at angles that avoid entry through excess normal tissue. In addition, beam shaping with multileaf collimators (MLCs) or other devices should be appropriate for a given target and surrounding OARs. This can be evaluated by directly visualizing each field using the beam’s eye view and is also based on 3D isodose lines overlaid on the computed tomography (CT) images. When treating an area in the neck or thorax, for example, one should ensure the beams are not entering through the shoulders/arms or exiting the oral cavity unnecessarily. For intensity-modulated radiation therapy (IMRT) plans, one should consider the number of fields and their point of entry through the body and fluence patterns. Assessing the field arrangement and collimation may subsequently become important if target volume coverage or OAR dose limits are not optimal and may be improved with additional fields or different beam entry angles.
The number of fields or arcs is also a key factor in the treatment time. A patient undergoing a palliative radiation treatment may not be able to lie on the treatment table for long periods, and a faster treatment may be preferable. Radiation oncologists should also consider that patient mobilization and internal organ motion are increased with longer treatment times.
Coverage
Initially to ensure coverage, the plan should be evaluated qualitatively by review of structure and isodose contours on images. The prescription isodose line should cover its corresponding PTV, and inadequate coverage or excessive dose spillage outside the PTV should be identified and evaluated.
Coverage is then commonly quantified using a dose-volume histogram (DVH) plot where relative (percent) or absolute dose in Gray (Gy) is displayed on the x-axis, and relative or absolute volume in cubic centimeters is displayed on the y-axis. Often coverage is considered adequate when at least 95% of the PTV is treated to the prescription dose or higher, although variations are acceptable depending on the case.
The DVH must be used with caution. The DVH cannot assess the appropriateness of the targets and OARs. The DVH could report 100% coverage of the PTV by the prescription dose, but the PTV could be delineated incorrectly. Alternatively, 95% PTV coverage may not be met, but there may be a compromise between PTV coverage and OAR constraints, with an accepted sacrifice in PTV coverage to avoid unacceptable toxicity to a surrounding critical OAR. Furthermore, there may be excessive dose spillage through structures not reported within the DVH. Because this information cannot be obtained from the DVH alone, we recommend evaluating the 3D graphical plan qualitatively before proceeding to the DVH.
Heterogeneity/Hot Spots
Heterogeneity refers to the variability in dose distribution throughout the plan, and includes examining the minimum PTV dose (cold spot) and the maximum dose both within and outside of the PTV (hot spots). In a conventionally fractionated IMRT plan, the acceptable minimum dose in the PTV is often around 95% with the maximum around 115% of the prescription dose. The heterogeneity in conventionally fractionated 3D plans is typically larger than it is for IMRT plans, and thus greater variability is acceptable in 3D plans while care is taken to limit hot spots near critical OARs. When unsure about the suitable values of heterogeneity parameters, many radiation oncologists reference published or experimental cooperative group protocols that list such values for the particular disease site being treated.
After determining the quantitative values of the cold and hot spots, it is critical to review their locations within the treatment plan. A hot spot within the GTV may be acceptable as opposed to it being in a critical OAR. Similarly, a cold spot at the edges of the PTV is preferred to it being within the GTV or CTV.
Organs at Risk
The first step in evaluating the OARs is to review the objectives assigned to the planner and identify the priority of these constraints. Certain OARs have critical dose thresholds beyond which severe toxicity may occur, and these constraints are not to be violated. For example, a firm constraint for the optic pathway or spinal cord may be much more important to prevent blindness or paralysis than objectives for the parotid gland or oral cavity.
When evaluating OARs, one should review both the DVH as well as the 3D graphic plan. The DVH provides an initial starting point to ensure the maximum dose, the mean dose, and the volume constraints are met. Again, the DVH does not provide information regarding the spatial distribution of dose. As such, it is helpful to review the graphic plan to identify the location of the critical isodose levels for each OAR. One may want to review the location of the 45 or 50 Gy isodose line in relation to the spinal cord, for example. Additionally, by reviewing the location of several critical isodose lines on the graphic plan, a secondary check can be performed to ensure all OARs encompassed within those isodose lines have been contoured.
The graphic plan is also essential to review if an OAR constraint is not being met. It may be that the PTV is encompassing part of the OAR, and to treat the PTV adequately, part of the OAR must be sacrificed. In this situation, the priority of the OAR should again be considered. For example, the PTV may need to be cropped to spare the spinal cord, whereas it may be necessary to treat a portion of the mandible to ensure the tumor volume is covered.
To find values for OAR dose constraints, the most commonly used source for late effects in conventional fractionation is the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) data.1 For hypofractionated regimens, the American Association of Physicists in Medicine (AAPM) TG-101 is also a valuable reference.2 Recent phase III protocols will also often specify planning objectives and acceptable variations with various levels of evidence supporting their use. Because constraints vary based on the dose per fraction, it is important to ensure appropriate values are used with biologically effective dose (BED) conversion when appropriate.
Prescription
The last step is to finalize and confirm the prescription. The dosimetrist may have edited the prescription after generating the plan, and one must ensure the total dose and dose per fraction are correctly entered. The treatment details must also be specified, including the type of radiation, energy, delivery method (3D, IMRT, enface, etc.), and delivery schedule (weekdays, every other day, twice daily, etc.).
The image guidance or setup verification imaging should be specified in the prescription. Image guidance requirements and techniques are at times specified by clinical protocols or are selected by the treating physician based on the size of the setup margin. In general, daily image guidance using cone-beam CT (CBCT) may be preferred when treating with smaller PTV margins at 3-5 mm. When treating with a larger margin in more difficult-to-immobilize areas, such as a palliative 3D bone metastasis plan with a 1 cm PTV margin, only portal imaging at the time of setup may be sufficient. In such cases, one must ensure that the PTV margins are appropriate for the image guidance technique, with smaller margins necessitating more frequent and accurate image guidance.
Conclusion
CB-CHOP is an effective acronym that provides a systematic, step-wise approach to analyzing multiple components of treatment plan quality (Figure 1
). An in-training radiation oncologist can use CB-CHOP as a foundation on which additional skills and thought processes can be built with further experience. Since plan approval is the critical step that transitions from cognitive processes to direct intervention with radiation therapy, CB-CHOP can provide a framework for a pre-intervention safety checklist, which has been shown to reduce errors and improve quality of care in other interventional disciplines.3 Treatment plan evaluation and approval remain the key responsibility of the physician and, thus, developing a consistent approach is a vital part of training. While current research is investigating objective, mathematical approaches to treatment plan evaluation, to our knowledge these techniques have not yet been implemented into daily clinical practice.4
A common pitfall in training or practice is relying on plans generated by a trusted, well-respected dosimetrist who has significant experience. However, mistakes happen, and dosimetrists change with time and institution. Since the final responsibility for a plan’s suitability lies with the radiation oncologist, it is important to remain thorough and objective with a standardized method to properly develop and implement plan evaluation skills.
Another key point is that it is common to request a plan revision to improve target coverage or OAR objective doses. While revisions may be requested repeatedly until an appropriate plan is generated, a threshold has been described beyond which further improvements in the plan are minimal and, in fact, may be detrimental due to the delay in initiating treatment.5 To proceed expeditiously, we suggest making all foreseeable requested changes at the first review. Use of the CB-CHOP framework may help serve as a checklist to ensure all potential areas of improvement are evaluated.
In summary, CB-CHOP is a memorable, simple approach that can be utilized to ensure key aspects of a radiation treatment plan are properly reviewed prior to plan approval and initiation of radiation treatment.
References
- Quantitative Analyses of Normal Tissue Effects in the Clinic. Int J Radiat Oncol Biol Phys. 2010;76(3):S1-160.
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078-4101.
- Haynes A, Weiser T, Berry W, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. NEJM. 2009;360:491-499.
- Ventura T, Lopes M, Ferreira B, et al. SPIDERplan: a tool to support decision-making in radiation therapy treatment plan assessment. Rep Pract Oncol Radiother. 2016;21:508-516.
- Moore K, Brame R, Low D, et al. Quantitative metrics for assessing plan quality. Semin Radiat Oncol. 2012;22:62-69.
Citation
M D, R J, E M, E F, R Y, R M. CB-CHOP: A simple acronym for evaluating a radiation treatment plan. Appl Radiat Oncol. 2017;(4):28-30.
December 14, 2017