Balanitis: An Unexpected Adverse Reaction to Pelvic Radiation or to Chemotherapy? Two Cases and a Review of the Literature
Images



Skin changes caused by ionizing radiation are one of the most common side effects of radiation treatment, with radiation dermatitis occurring in about 85% to 95% of cancer patients receiving radiation treatment.1 Severity can range from mild erythema to moist desquamation and ulceration.
Radiation is often combined with chemotherapy for concurrent chemoradiation treatment. As a result, it can often be difficult to distinguish whether an adverse reaction to treatment is due to radiation or chemotherapy. It is important to properly identify the causative agent for an adverse reaction, and failure to do so may not only worsen patients’ quality of life, but also delay or interrupt treatment.
Generally, when patients have a skin reaction near the area of radiation treatment it is attributed to radiation. However, closer inspection may show this is not always the case. Here, we present 2 cases of patients receiving concurrent chemoradiation to the pelvis who developed blistering at the head of the penis. The immediate assumption was that this adverse reaction was caused by radiation. Further review of the treatment plan and the literature suggest that this rare adverse effect—also known as balanitis, inflammation of the head of the penis—is associated with chemotherapy, specifically 5-FU and capecitabine.2-5
CASE SUMMARIES
Patient 1
Patient 1 is a 59-year-old man with a diagnosis of T3N0M0 rectal cancer. He underwent a screening colonoscopy examination in 2009 and was found to have cancer in situ in a resected polyp in the rectum. He did well until August 2019 when a follow-up screening colonoscopy examination identified a 1.5-cm sessile lesion at about 10 cm from anal verge; biopsy confirmed invasive carcinoma. A computed tomography (CT) scan of the chest/abdomen/pelvis showed no evidence of distant metastases. MRI of the pelvis showed a mass-like lesion over the proximal and midrectum with a cranial caudal extension about 2.4 cm involving the proximal and midrectum with transmural involvement and without evidence of enlarged lymphadenopathy. Based on imaging findings, his rectal cancer was considered T3N0M0.
Patient 1 was offered total neoadjuvant therapy followed by surgery to treat his rectal cancer. He was initially started on CAPEOX, but he was unable to tolerate capecitabine due to an adverse skin reaction on his hands and feet, and CAPEOX was stopped after 2 cycles. His chemotherapy was subsequently changed to FOLFOX for 4 more cycles, which the patient tolerated well. A follow-up CT scan of his chest, abdomen, and pelvis showed no evidence of distant metastases. Patient 1 then underwent chemoradiation with infusional 5-FU (2,250 mg weekly) and 54 Gy in 30 fractions to the pelvis using a 4-field box technique.
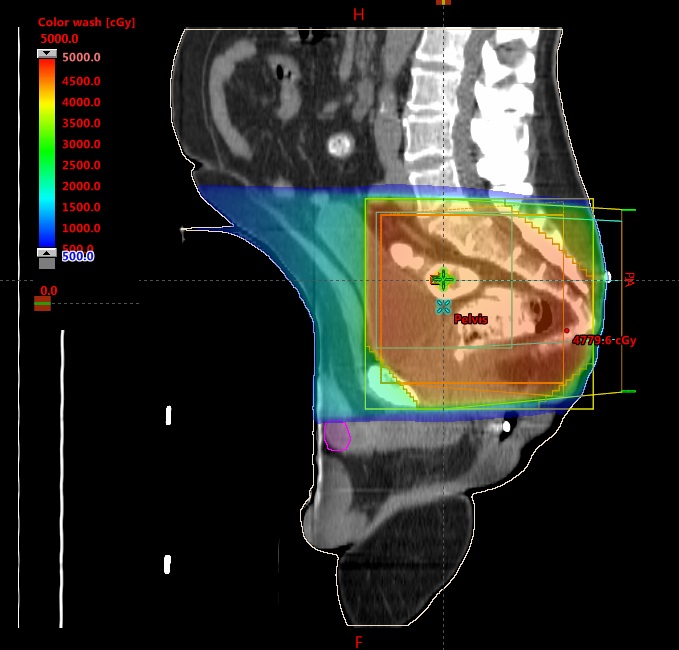
Around week 5 out of 6 of radiation treatment (after 41.4 Gy out of 54 Gy), he was noted to have erythema with minor blistering lesions over the head of the penis. There was some concern that this may be an adverse reaction to radiation, but reviewing his radiation treatment plan did not show significant radiation dose to the head of the penis (Figure 1). Further literature review showed an association between 5-FU and inflammation at the head of the penis. His medical oncologist was informed, and it was decided to continue treatment and monitor. He was able to complete the rest of treatment without interruption, although his penile toxicity did not improve until after completion of treatment. MRI and endoscopic evaluation 3 months post-treatment showed that the patient achieved clinical complete response and he is currently on a “watch and wait” protocol.
Patient 2
Patient 2 is a 41-year-old man with a diagnosis of T3N1M0 rectal cancer. He initially presented with changes in bowel habits, intermittent rectal bleeding, and urgency for several months. He eventually underwent an esophagogastroduodenoscopy (EGD) and colonoscopy in early 2020, which showed a circumferential mass approximately 12 cm from the anal verge, which biopsy confirmed to be invasive carcinoma. The tumor was obstructing and could not be passed. Patient 2 underwent a staging CT scan of the chest, abdomen, and pelvis, which showed no evidence of metastatic disease. He also underwent an MRI of the pelvis, which was concerning for possible regional adenopathy. Based on imaging findings, his rectal cancer was considered T3N1M0.
Patient 2 was offered total neoadjuvant therapy followed by surgery to treat his rectal cancer. He completed 8 cycles of FOLFOX without any issues. Follow-up CT scan of his chest, abdomen, and pelvis showed no evidence of distant metastases. Patient 2 then underwent chemoradiation with capecitabine (2,000 mg twice daily) and 54 Gy in 30 fractions to the pelvis using intensity- modulated radiation therapy (IMRT).
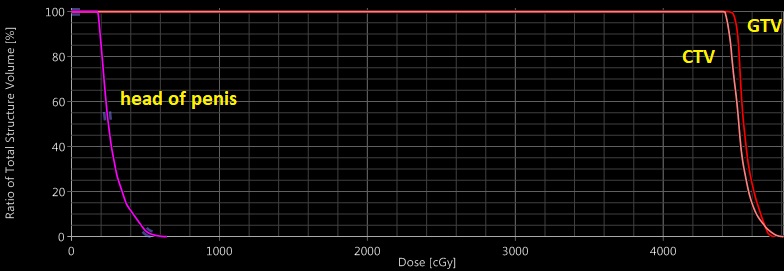
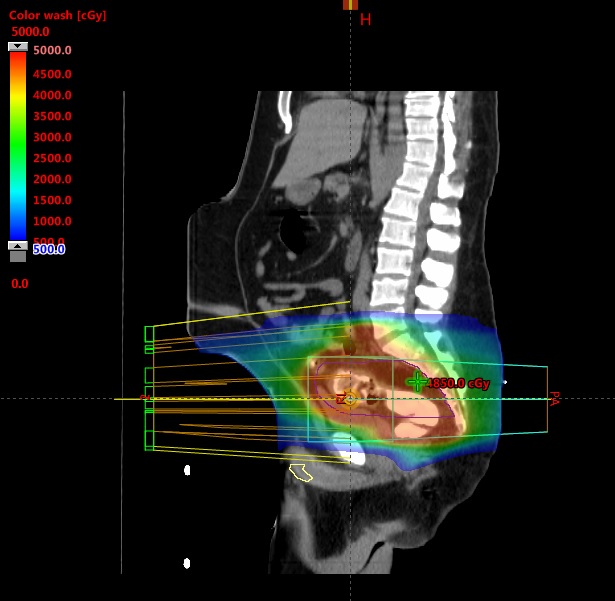
Around week 5 out of 6 of radiation treatment (after 39.6 Gy out of 54), he developed blistering over the head of the penis (Figure 2). Again, reviewing his radiation treatment plan did not show any radiation dose to the penis (Figure 3). Further literature review showed an association between capecitabine and inflammation at the head of the penis. His medical oncologist was informed, and his dose of capecitabine was decreased from 2,000 mg twice daily to 1,500 mg twice daily. Patient 2 was able to complete the rest of treatment without interruption and is currently pending evaluation to assess his response after therapy. Surgical intervention will be followed.
DISCUSSION
One of the most common skin reactions caused by capecitabine and 5-FU is hand-foot syndrome, also known as palmar-plantar erythrodysesthesia. This reaction mostly affects the palms of the hands and soles of the feet, causing inflammation and blistering. Other cytotoxic drugs linked to hand-foot syndrome include pegylated liposomal doxorubicin, docetaxel, vinorelbine, gemcitabine, and sorafenib.6 Severity can range from National Cancer Institute (NCI) grade 1 (dermatitis, redness, swelling, and hyperkeratosis without pain) to NCI grade 3 (peeling, blisters, bleeding, fissures, swelling, hyperkeratosis with pain, and limiting self-care activities).7
Generally, the management for hand-foot syndrome is delaying treatment for up to 2 weeks until symptoms resolve to grade 0-1.8 Local supportive measures such as cooling and moisturizing the affected areas have been shown to be helpful.9 Systemic therapy may be considered for severe cases, with systemic corticosteroids and pyridoxine showing varying degrees of efficacy in treating hand-foot syndrome.10 Symptoms typically resolve within 2 to 4 weeks.
While hand-foot syndrome is a well-known side effect of capecitabine and 5-FU, manifestations outside the hands and feet are uncommon. Skin toxicity involving the genitals is even less common, and only a few case reports document this rare adverse reaction.2-5 Upon reviewing the literature, we have identified 2 cases of 5-FU-associated balanitis and 2 cases of capecitabine-associated balanitis. Table 1 summarizes reported cases of chemotherapy-induced balanitis including the patients in this case series to better represent how this unexpected skin toxicity may present.
Based on the literature, it appears that most cases of balanitis are grade 1 to 2 with a symptom onset between 2 to 16 weeks after initiating treatment. All cases of balanitis resolved 2 weeks after decreasing the dose or taking a break from the offending agent. The 2 cases we present in this case report are consistent with previous case reports.
We acknowledge that the penis is a free mobile organ, and the case report findings could be limited by the fact that the penis could be in various positions (in or outside the radiation fields) during daily setup. However, examining the sagittal views in Figures 1 and 3 show that even with the penis head in the most superior position, it is unlikely to receive any more than 5-10 Gy based on the dose color wash. Also, for patients being treated with IMRT, daily imaging with cone-beam CT (CBCT) could help in tracking the penile tissue during daily treatment. Patient 2 was treated with IMRT, and after he developed balanitis, we actively tracked his penile tissue with CBCT to see if it was inside the radiation field.
Several strategies can help radiation oncologists manage balanitis whether it is caused by chemotherapy or radiation. For mild dermatitis and focal pruritis, skin care products such as Aquaphor and Eucerin cream may be used. For more severe moist desquamation, skin care products containing antibiotics such as Silvadene may be used. For daily set-up considerations, if the patient is receiving IMRT, checking to see whether the head of the penis is in the field of radiation with CBCT is important. As always, it is important to communicate with the medical oncologist to see if any treatment-related issues are related to the chemotherapy or radiation.
CONCLUSION
Balanitis is a rare skin toxicity associated with chemotherapy. For patients receiving concurrent chemoradiation, especially to the pelvic area, it can be easy to misattribute the cause of balanitis to radiation. Incorrectly attributing the cause of this adverse reaction may worsen patient outcomes and prolong patient suffering. It is thus important to showcase these two cases to help clinicians make more informed decisions when encountering these types of reactions in their practice.
REFERENCES
- Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 Pt 2):985-993. doi:10.1038/jid.2011.411
- Tas F, Basaran M, Aydiner A, Eralp Y, Topuz E. 5-Fluorouracil-induced balanitis in a patient with oesophageal carcinoma. Clin Oncol. 2001;13(3):170-171. doi:10.1053/clon.2001.9247
- Sapp CM, DeSimone P. Palmar-plantar erythrodysesthesia associated with scrotal and penile involvement with capecitabine. Clin Colorectal Cancer. 2007;6(5):382-385. doi:10.3816/CCC.2007.n.008
- Micevic G, Perkins SH, Zubek AE. Balanitis associated with FOLFIRI chemotherapy. JAAD Case Rep. 2018;4(1):58-60. doi:10.1016/j.jdcr.2017.09.001
- Hu H, Corkum MT, Perera F. Palmar-plantar erythrodysesthesia with genital involvement secondary to capecitabine chemoradiotherapy: a case report. Cureus. 2018;10(12):10-15. doi:10.7759/cureus.3704
- Farr KP, Safwat A. Palmar-plantar erythrodysesthesia associated with chemotherapy and its treatment. Case Rep Oncol. 2011;4:229-235. doi:10.1159/000327767
- Webster-Gandy JD, How C, Harrold K. Palmar-plantar erythrodysesthesia (PPE): a literature review with commentary on experience in a cancer centre. Eur J Oncol Nurs. Published online 2007. doi:10.1016/j.ejon.2006.10.004
- Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1990;40(3)367-398. doi:10.1016/S0190-9622(99)70488-3
- Mangili G, Petrone M, Gentile C, de Marzi P, Viganò R, Rabaiotti E. Prevention strategies in palmar-plantar erythrodysesthesia onset: the role of regional cooling. Gynecol Oncol. 2008; 108(2):332-335. doi:10.1016/j.ygyno.2007.10.021
- Chen M, Zhang L, Wang Q, Shen J. Pyridoxine for prevention of hand-foot syndrome caused by chemotherapy: a systematic review. PLoS ONE. 2013;8(8):e72245. doi:10.1371/journal.pone.0072245
Citation
J L, YJ C. Balanitis: An Unexpected Adverse Reaction to Pelvic Radiation or to Chemotherapy? Two Cases and a Review of the Literature. Appl Radiat Oncol. 2020;(4):45-48.
December 24, 2020