A Practical Method to Prolong Expiratory Breath Holds for Abdominal Stereotactic Body Radiation Therapy
Images
Introduction
Stereotactic body radiation therapy (SBRT) is an increasingly utilized radiation technique that enables accurate delivery of ablative radiation doses with a steep dose fall-off to surrounding tissues. However, the utilization of SBRT for moving targets can be a significant challenge. Failure to account for respiratory motion can lead to underdosing targets and overdosing normal tissues.1,2 Motion management is especially important for abdominal SBRT cases given the high dose per fraction and steep dose gradients between the tumor target and nearby gastrointestinal viscera (such as the stomach and the bowel). Various strategies have been utilized to minimize the effects of respiratory motion during abdominal/thoracic SBRT, such as abdominal compression, amplitude- and phased-based gating, and breath-hold techniques, among others.1,3-5
Voluntary breath-hold techniques are attractive for motion management during abdominal SBRT. With this strategy, the beam is intermittently enabled only when the patient is holding their breath, which is coordinated via instructions from the radiation therapists. As the tumor and target tissue are effectively stationary during beam-on, there is no need for an internal target volume margin, which minimizes the volume of irradiated normal tissue to achieve adequate tumor target coverage. This reduction in motion translates to improved on-board cone-beam CT (CBCT) image quality, allowing more accurate patient alignment prior to SBRT treatment.6-8
Both inspiratory breath-hold (IBH)5,9,10 and expiratory breath hold (EBH)11-13 techniques have been successfully utilized for SBRT treatments. While EBH is more reproducible and minimizes target motion compared with IBH,13-19 it is generally more challenging for patients to perform an EBH of sufficient duration compared with IBH.20 Physiological studies of breath holding have shown that supplemental oxygen and mild hyperventilation can significantly improve breath-hold durations,20 with each technique adding incrementally to the improvement in breath-hold duration. Several pilot studies have demonstrated the clinical effectiveness of supplemental oxygen and mild hyperventilation for deep-inspiratory breath hold (DIBH) treatment in patients with breast cancer,21-23 but the effectiveness of this technique for patients undergoing EBH is unknown.
In this article, we report the experience of the first 20 patients treated with abdominal SBRT using a supplemented EBH technique (EBHsupp) with supplemental oxygen and mild hyperventilation. We evaluated data on individual patient EBH durations and treatment times, and we compared this data with a cohort of similar patients treated with EBHs without supplementation (EBHRA, room air, no mild hyperventilation). We hypothesized that the EBHsupp technique would prolong EBHs and reduce overall treatment time compared with EBHRA.
Materials and Methods
Patient Inclusion/Exclusion Criteria
All patients receiving 3-fraction abdominal SBRT treated with an EBH technique in our department from January 2018 onward receiving between 1300 cGy and 1500 cGy per fraction were included (Institutional Review Board 120703005). Other SBRT fractionation schemes were not included to reduce heterogeneity in treatment characteristics that might influence overall treatment time (eg, reduced monitor units [MUs] per treatment for 5-fraction plans or for 3-fraction plans with lower prescription doses, increased patient practice/experience with 5-fraction treatments). Patient demographic and clinicopathologic information (age, gender, diagnosis, and comorbidities) were obtained from the medical record, and treatment details (dose per fraction, number of treatment arcs, MUs delivered per treatment) were obtained from the oncology information system (OIS) (ARIA; Varian).
Treatment Procedure Details
Prior to August 2020, patients were treated with standard EBH without oxygen supplementation or coaching/prompting of their respiratory rate (RR) prior to EBH (designated EBHRA, where RA signifies room air). After August 2020, as part of a quality improvement initiative in our department and after a successful proof-of-concept study in healthy volunteers (see
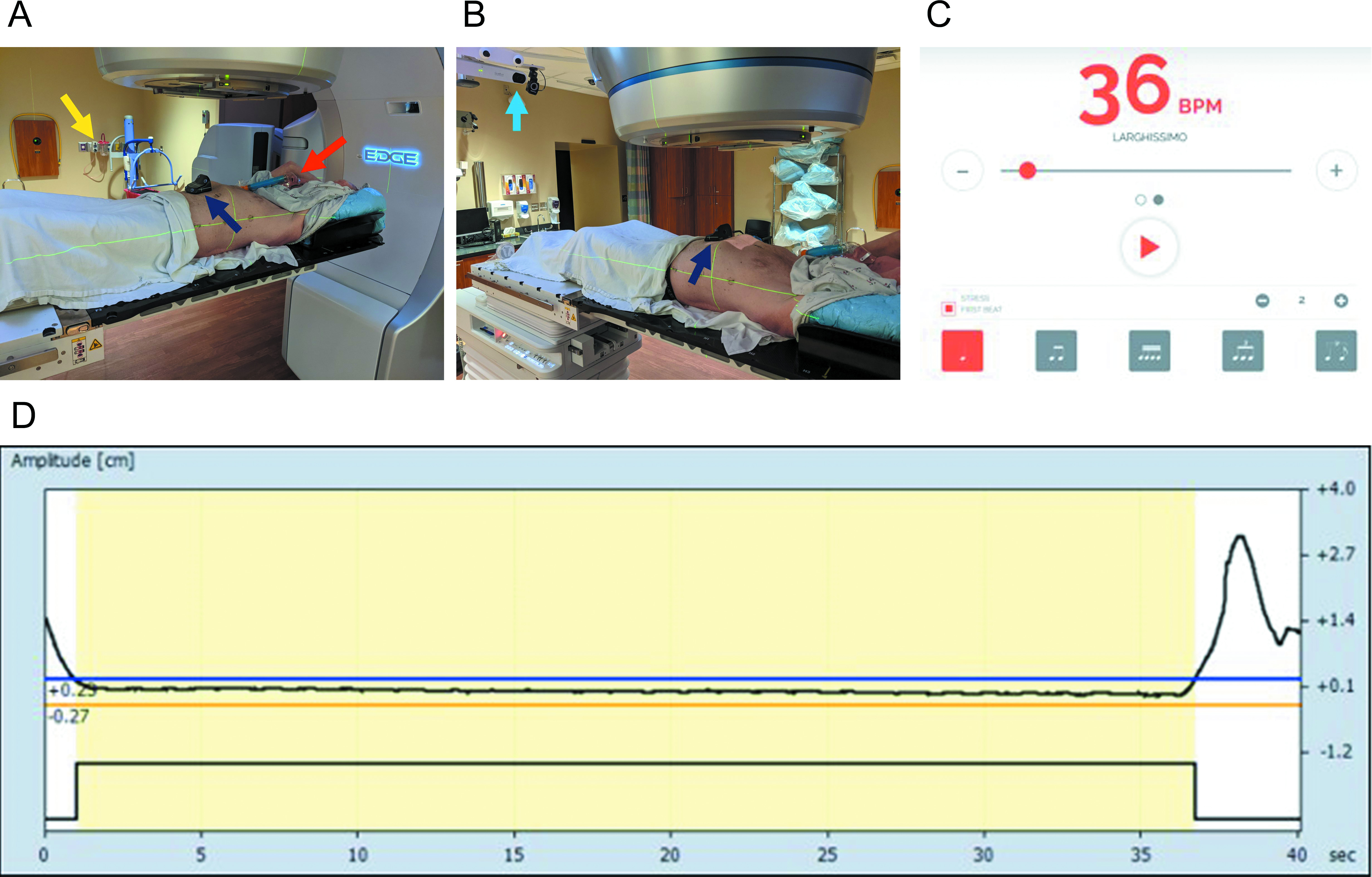
Implementation details of the supplemented expiratory breath hold technique (EBHsupp). Supplemental oxygen was delivered at 50% FiO2 via a Venturi mask and appropriate adapter (red arrow) connected to in-house oxygen flowmeters (yellow arrow) in the treatment delivery vault (A). Varian’s Real-Time Position Management (RPM) system was used to track patient respiratory motion with an infrared tracking camera (light blue arrow) and reflective marker (dark blue arrow) placed on the patient’s abdomen (B). Patients were encouraged to breathe at a respiratory rate of 18 breaths/min with the use of an online metronome. The metronome was set at 36 beats/min with stress on the first beat so there would be a distinct sound cue for both the start of inhalation and the start of exhalation (C). An example of breath-hold tracing during patient treatment via the RPM system is shown (D). Permission was given by a patient volunteer for the use of photographs in this article.
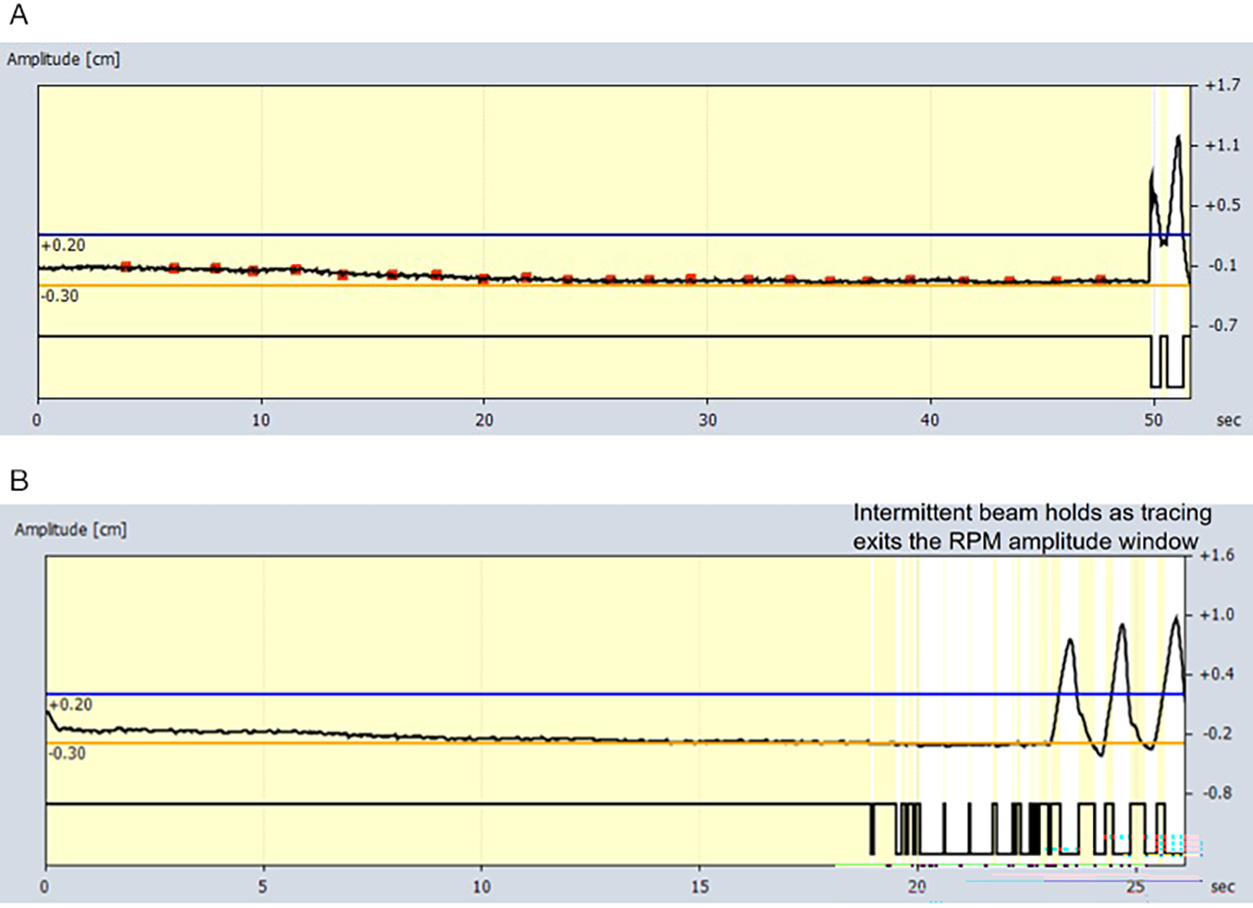
For both EBHRA and EBHsupp, patients were screened at the time of CT simulation, per standard departmental protocol, to verify their ability to perform repeated EBHs of more than 20 seconds’ duration. This was assessed by radiation therapy staff with the patient on the CT simulation table and in the treatment position with full immobilization gear and tracking of abdominal excursion via Varian’s Real-Time Position Management system (RPM). Completion of 2 consecutive, 20+ second EBHs within a 5-mm-amplitude window (as tracked by RPM) was required for the patient to move forward with EBH CT simulation and treatment. Patients not able to complete EBH simulation were treated with phased-based respiratory gating and are not described in this article. To be eligible for EBH-based SBRT treatments at our institution, all patients (both for EBHRA and EBHsupp) were required to have either implanted fiducials or radio-opaque transarterial chemoembolization (TACE) material within or directly adjacent to the target to allow for intrafraction kV x-ray real-time monitoring of motion in addition to RPM amplitude gating.
CT Simulation and Target Delineation
Following EBH CT simulation, contouring and planning were performed on the EBH CT simulation scans. Gross tumor volumes (GTVs) were defined on the EBH CT simulation scan with assistance from fused diagnostic images (eg, triple-phase MRI). For post-TACE targets without residual enhancing tumors, a clinical target volume (CTV) encompassing the TACE volume was contoured in lieu of a GTV. Otherwise, CTV was a 3- to 4-mm isotropic expansion from the GTV with cropping at natural boundaries (eg, edge of the liver). The planning target volume was generated via an isotropic 5-mm expansion of the CTV. Tracking structures (fiducials or TACE) were contoured with the bone window. Two planning organ-at-risk (PRV) volumes were generated for these tracking structures via 3-mm and 5-mm isotropic expansions for PRV3 and PRV5 structures, respectively, to allow for intrafraction kV assessment of tracking structure displacement during treatments. Volumetric-modulated arc therapy (VMAT) plans consisting of 2 or 3 coplanar arcs were generated for all patients.
Treatment Delivery
On the days of treatment, the general treatment workflow for both EBHRA and EBHsupp treatments was as follows (see
Treatment timeline example and definition of time points. The shaded gray area shows an example “Session Timeline” screenshot from Varian Eclipse treatment planning software from a patient treated with a supplemented expiratory breath-hold (EBH) technique. The individual EBHs performed by the patient during cone-beam CT (CBCT) and delivery of treatment arcs are shown in the amplitude tracings at the top of the figure (EBH#1 to EBH#6). Treatment time definitions are shown toward the bottom of the figure (and are described in the “Materials and Methods” section).
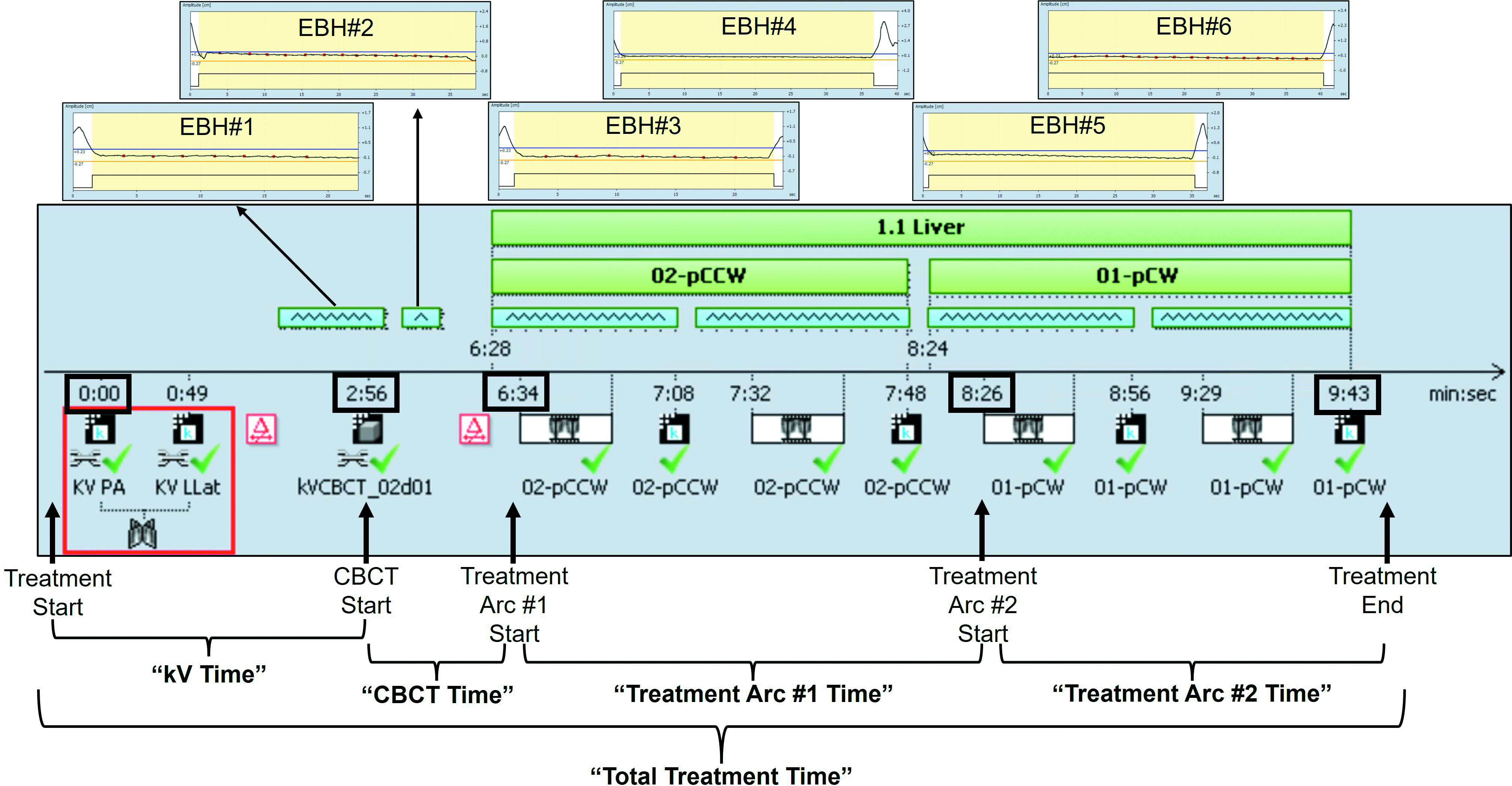
The RPM amplitude gating (5 mm) and intrafraction fiducial/TACE tracking with triggered kV images obtained every 10/20 degrees of gantry rotation were used to confirm breath-hold position during treatment (see
Data Extraction
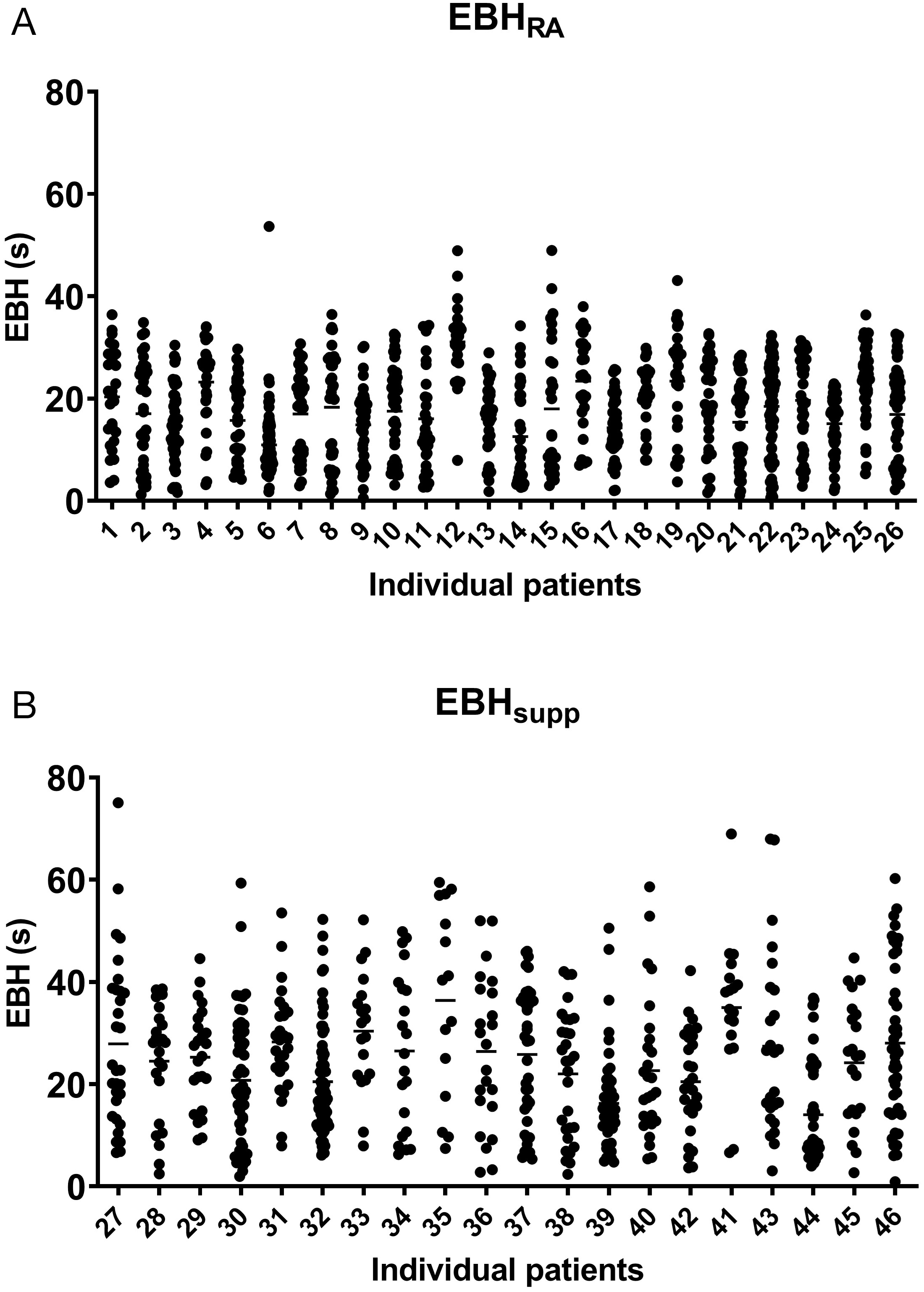
Individual patient EBH duration data for every breath hold for all treatments were extracted from the OIS in text file form, including beam on/off times during EBH (
Statistical Analysis
Patient demographic, clinical, and treatment characteristics were compared between the EBHRA and EBHsupp groups via Student
Results
We identified a total of 46 patients meeting inclusion criteria who received 3-fraction SBRT with EBH treatment in our department after January 2018. Prior to the initiation of the EBHsupp technique in August 2020, 26 patients were treated with 28 treatment plans via standard EBHRA (2 patients had 2 liver tumors that were treated with separate treatment plans), accounting for a total of 83 EBHRA treatments (28 treatment plans × 3 fractions = 84 treatments minus 1 patient who received a liver transplant after the completion of only 2 fractions). At the time of this analysis, 20 patients were treated with the EBHsupp technique with 24 treatment plans (4 patients had 2 liver tumors who were treated with separate treatment plans) for a total of 72 EBHsupp treatments (24 treatment plans × 3 fractions = 72 treatments).
Patient demographic, clinicopathologic, and treatment parameters are shown in
Patient Clinicopathologic Characteristics and Relevant Treatment Parameters
| VARIABLE | EBHRA( |
EBHsupp( |
|
|---|---|---|---|
| Age (mean {SD}) | 62.4 {11.1} | 63.2 {10.0} | .7881 |
| Gender | |||
| 6 (23.1) | 2 (10.0) | .246 | |
| 20 (76.9) | 18 (90.0) | ||
| Diagnosis | |||
| 19 (73.1) | 15 (70.0) | .883 | |
| 1 (3.8) | 1 (5.0) | ||
| 6 (23.1) | 4 (20.0) | ||
| CCI (mean {SD}) | 7.31 {1.52} | 7.60 {2.14} | .590 |
| Current smoker (%) | 9 (34.6) | 5 (25.0) | .482 |
| Dose per fraction (cGy) | .415 | ||
| 3 (11.5) | 5 (25.0) | ||
| 1 (3.8) | 0 (.0) | ||
| 22 (84.6) | 15 (75.0) | ||
| Number of treatment arcs | .099 | ||
| 26 (100.0) | 18 (90.0) | ||
| 0 (.0) | 2 (10.0) | ||
| MU per treatment (mean {SD}) | 4162 {967} | 4058 {950} | .695 |
For continuous variables, the mean value and standard deviation are shown. For categorical variables, the number of patients is presented, with the number in parentheses representing the percentage of patients. >P values were calculated via Student t-test and the χ2 test for continuous and categorical variables, respectively.
Abbreviations: CCI, Charlson comorbidity index; EBH, expiratory breath hold; MU, monitor units; RA, room air.
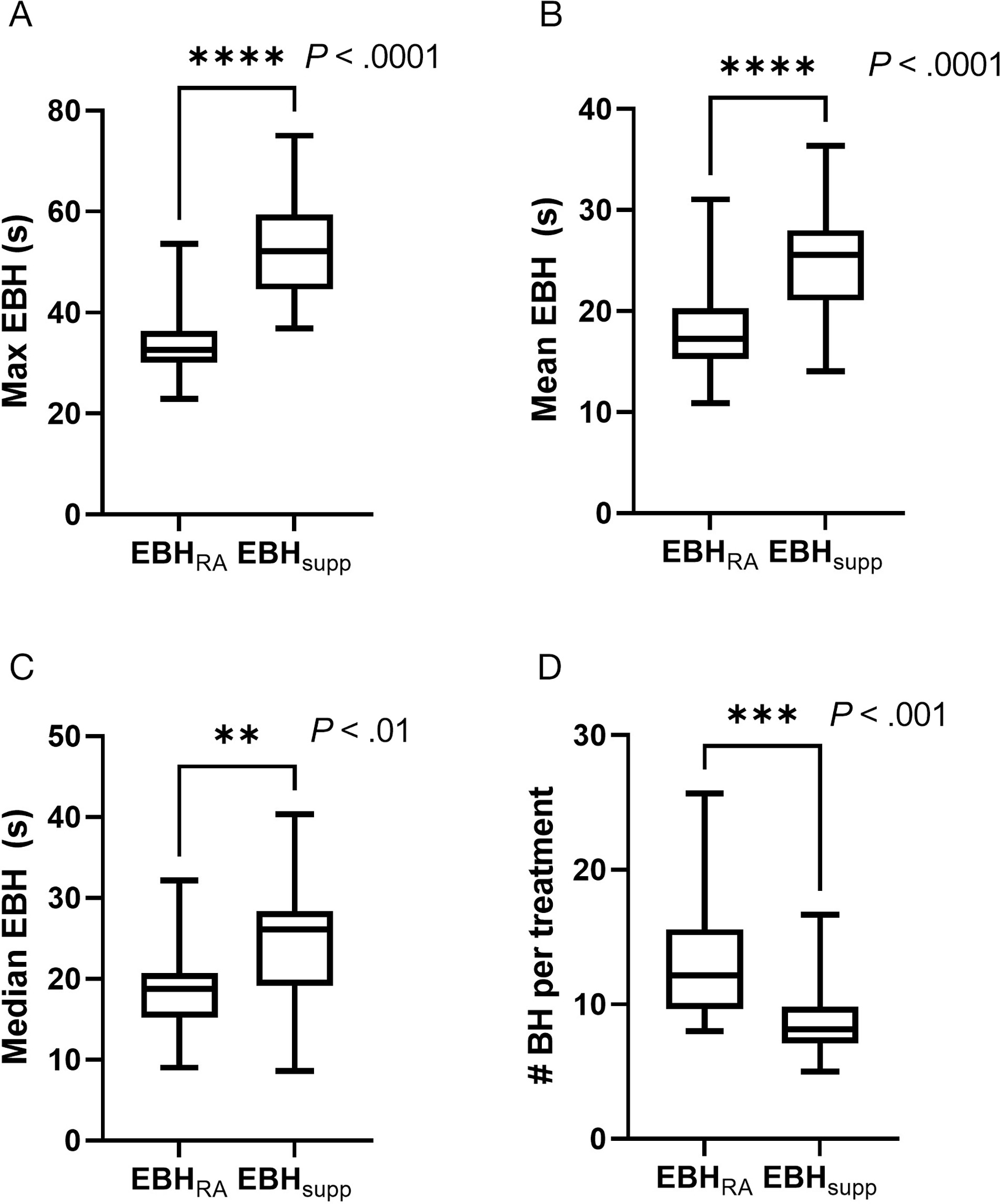
A total of 1735 individual EBHs were extracted from the OIS (
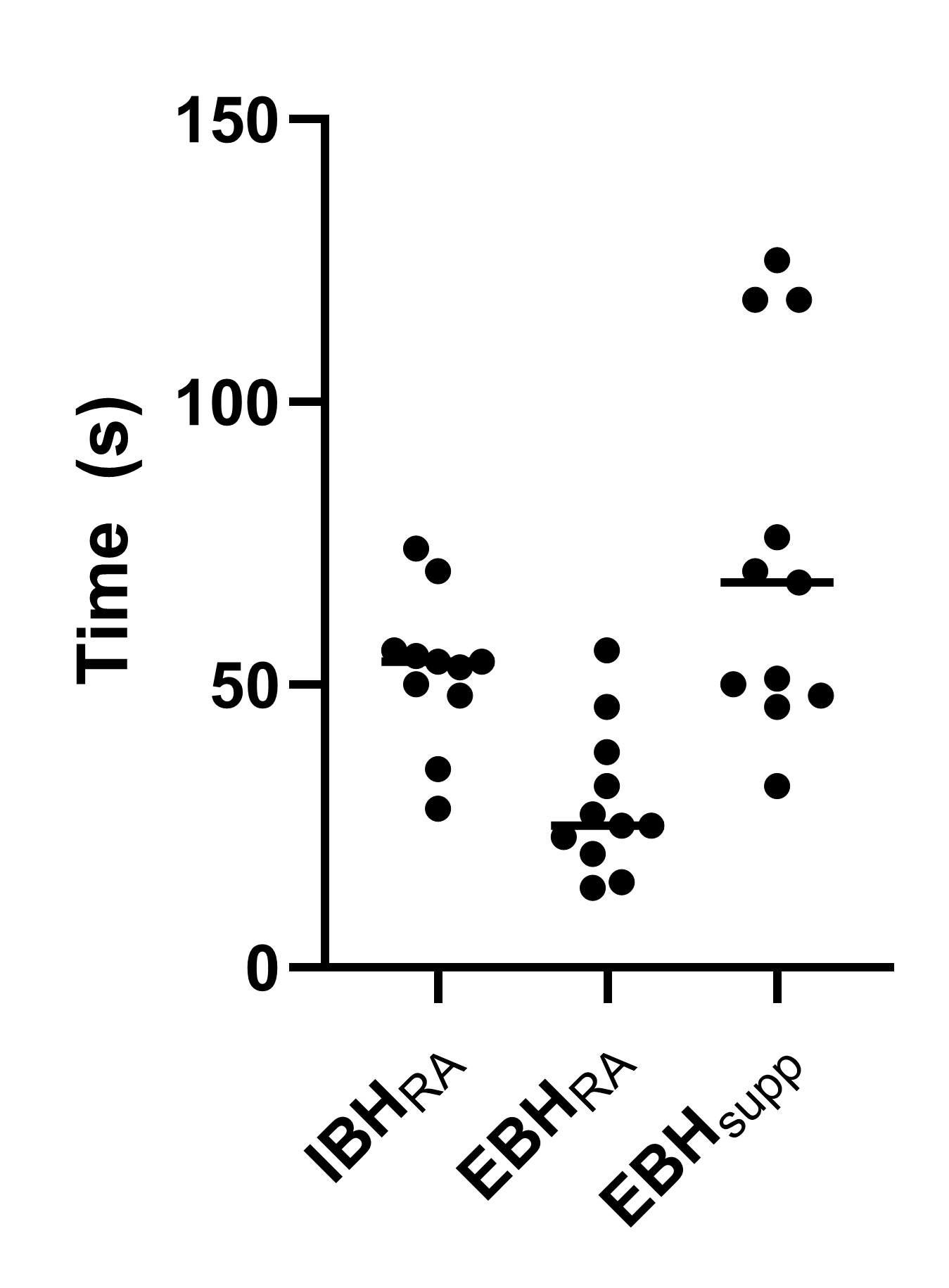
Duration and number of individual expiratory breath holds (EBHs) during stereotactic body radiation therapy treatments. The data illustrate the average maximum (Max EBH) (A), mean (Mean EBH) (B), median (Median EBH) (C), expiratory breath-hold duration of individual patients, as well as the average of the total number of expiratory breath holds required for individual patients to complete treatment for patients treated with standard (EBHRA) or supplemented (EBHsupp) EBH technique (D). Error bars in the box and whisker plot represent the range (min to max), bars represent the interquartile range, and line represents the median. P values were calculated via a Student t-test.
Descriptive Statistics of Expiratory Breath-Hold (EBH) Times
| STATISTIC | EBHRA (s) | EBHsupp (s) | |
|---|---|---|---|
| 10th percentile | 6.4 | 7.8 | .219 |
| 25th percentile | 11.3 | 14.7 | .031 |
| 50th percentile | 18.7 | 24.9 | .002 |
| 75th percentile | 24.6 | 34.7 | <.001 |
| 90th percentile | 29.3 | 43.1 | <.001 |
| Max | 34.5 | 52.8 | <.001 |
| Mean | 18.2 | 25.1 | <.001 |
Values reported are the mean values for each EBH statistic computed for patients in the control group (EHBRA) and the supplemented group (EBHsupp). P values reported were calculated via Student t-test.</em
Abbreviation: RA, room air.
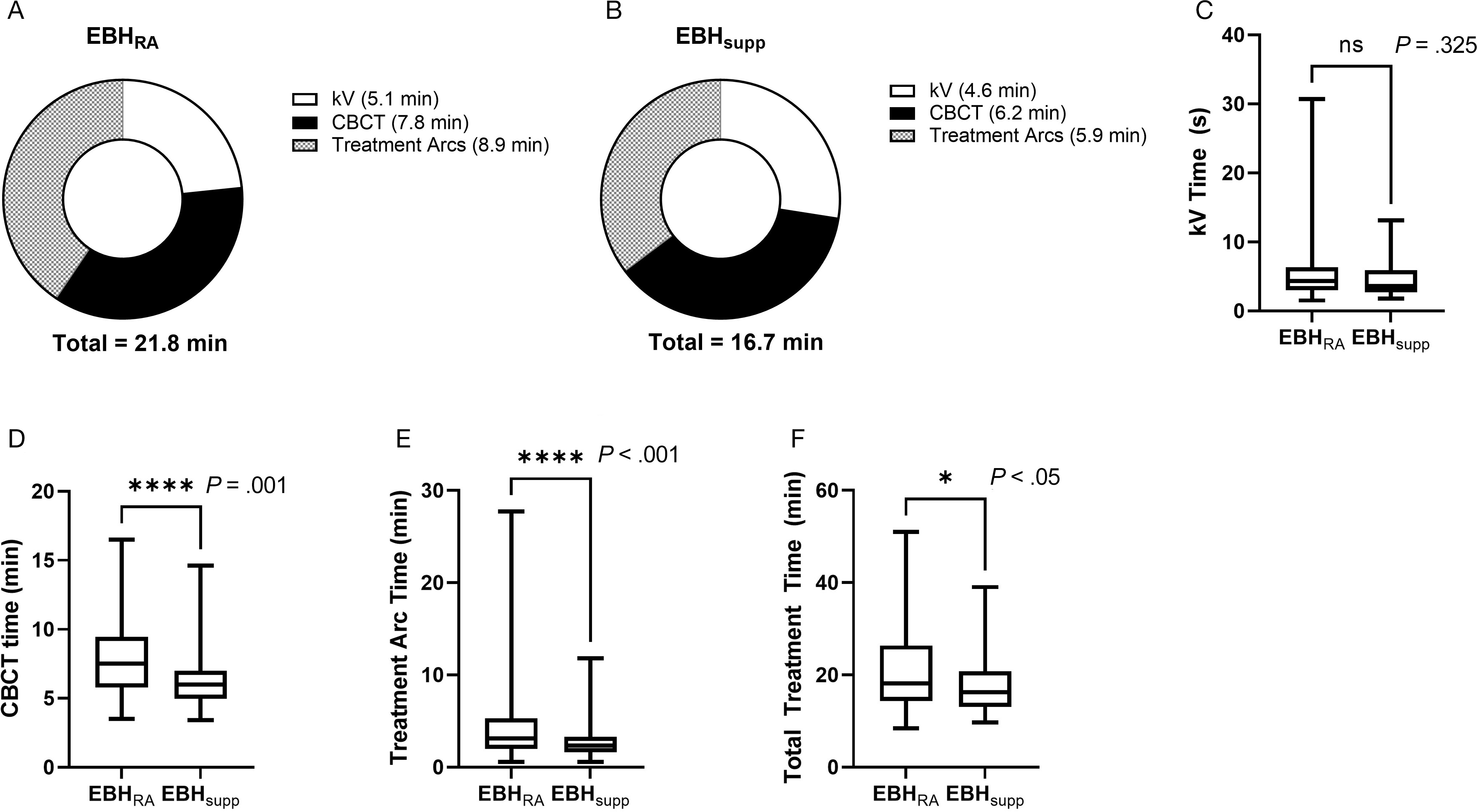
Examination of treatment times between the 2 groups (
Times for completion of individual treatment components and for total treatment. Pie charts showing the mean times required for each component of treatment and the total treatment time for individual patients treated with standard expiratory breath hold (EBH) (EBHRA) (A) or supplemented EBH (EBHsupp) techniques (B). Box and whisker plots of time to completion of orthogonal kVs (kV Time) (C), time to completion of cone-beam CT (CBCT Time) (D), time to completion of treatment arcs (Treatment Arc Time) (E), and total treatment time (F). Error bars in the box and whisker plot represent the range (min to max), bars represent the interquartile range, and line represents the median. P values were calculated via Mann-Whitney U test.
Discussion
Expiratory breath hold is an effective method for motion management in patients undergoing abdominal SBRT treatments but is underutilized due to patients’ difficulty in performing repeated EBH of sufficient duration.1 In this study, we found that patients undergoing abdominal SBRT with supplemental oxygen and mild hyperventilation exhibited prolonged EBH durations compared with patients treated with nonsupplemented EBH. Patients in the EBHsupp group performed EBH of longer duration by all reported metrics (maximum EBH, mean EBH, and median EBH) and required less total EBH to complete treatments. These results agree with previously published experiences utilizing similar techniques in patients undergoing DIBH for breast cancer treatments20-22 as well as a recent randomized study of volunteers undergoing EBH that showed an improvement in median EBH duration from 24 seconds to 49 seconds with supplemental oxygen and mild hyperventilation.24
The reduction in treatment times observed in the EBHsupp group compared with the EBHRA group is likely related to improve EBH using the supplemented technique. This is supported by significant improvements in time to complete tasks that required prolonged EBH (eg, CBCT, treatment arcs) and a lack of significant improvement in tasks that did not require prolonged EBH (eg, kV acquisition/alignment). There were no significant differences in patient clinical/pathological characteristics between the groups (eg, age, diagnosis, and comorbidity index) nor in treatment-related parameters that might be expected to influence treatment time (eg, dose per fraction, MU delivered, and number of treatment arcs). During the study period, there were no other changes in departmental protocols or treatment-planning techniques as an alternate explanation for the reduction in treatment time observed. While the EBHsupp technique reduced treatment times in our study, there was an initial time investment (~10-15 min) at the time of CT simulation for added patient training for breathing with a metronome and the use of a Venturi mask.
The reported EBHsupp technique was safe in our study population. We had no patients who had any issues or symptoms related to the breath-hold component of their treatments (eg, no lightheadedness, syncope, tingling, tetany, or other concerns), which is in agreement with other studies that have shown the safety of breath holds (both inspiratory and expiratory) with mild hyperventilation and supplemental oxygen.20-25 The mild hyperventilation used in this study was chosen based on the RRs previously utilized and found to be safe in breast cancer patients undergoing DIBH treatment with prolonged hyperventilation.22,23 We specifically avoided more rapid hyperventilation given the theoretical increased risk of tetany that can occur with more aggressive hyperventilation and associated hypocapnia.26 Similarly, the level of supplemental oxygen of 50% FiO2 was selected based on safety as supplemental oxygen levels above 60% are associated with an increased risk of absorptive atelectasis.25,27-30
One safety issue that should be mentioned for all breath-hold treatments (not just supplemented ones such as the EBHsupp method) is the well-established increase in blood pressure during prolonged breath holds.25,31,32 However, the risk and severity of blood pressure rise during breath holds do not appear to be worsened by supplemented techniques, including a similar technique with mild hyperventilation and supplemental oxygen.25 Further, a recent randomized study of EBHs in volunteers did not find a significant change in blood pressure during EBH, possibly due to the relatively modest prolongation of EBHs with supplemented techniques (< 1 min) compared with DIBH (up to 5 min prolonged breath holds reported).24 In general, patient cardiopulmonary comorbidities should be considered by the treating radiation oncologist prior to proceeding with breath-hold treatment with discussion of the technique with the patient’s other involved physicians (eg, cardiologist and pulmonologist) for those with significant cardiopulmonary comorbidities.
There are several limitations to this study. First, this was not a randomized trial and. therefore, there is a possibility of biases (eg, selection bias) that could have partially influenced the results between the EBHRA and EBHsupp groups, though patient groups were well balanced overall with respect to both clinical parameters and treatment parameters. Second, all the patients described here received liver SBRT and, therefore, the results may not be generalizable to all patients receiving abdominal SBRT, though we have additionally treated several patients with primary pancreatic cancer with pancreas SBRT with the EBHsupp method with similar experience to those treated with liver SBRT. Lastly, this was a “real-world” study of EBHsupp implementation in a busy radiation oncology clinic, and we did not attempt to capture nor control all aspects of respiratory physiology that govern breath-hold capacity. While we encouraged patients to breathe at a rate that would normally correspond to mild hyperventilation, we did not forcibly control patient RR or tidal volumes and did not measure partial pressures of carbon dioxide; therefore, whether hyperventilation/hypocapnia was achieved for each patient is unknown. Future mechanistic studies of patients undergoing repeated, supplemented EBH with real-time measurement of these parameters will be helpful in further optimizing supplementation/hyperventilation protocols and ensuring uniformity of technique among individual patients.
Conclusions
Patients receiving supplemental oxygen and mild hyperventilation exhibited prolonged EBH time and reduced overall treatment time during abdominal SBRT. This intervention is simple, inexpensive, safe, and may improve individual patient breath-hold times, reduce treatment time, and increase the number of patients eligible for EBH-based abdominal SBRT.
References
- Brandner ED, Chetty IJ, Giaddui TG, Xiao Y, Huq MS Motion management strategies and technical issues associated with stereotactic body radiotherapy of thoracic and upper abdominal tumors: a review from NRG oncology. Med Phys. 2017; 6(44):2595-2612
- Caillet V, Booth JT, Keall P IGRT and motion management during lung SBRT delivery. Phys Med. 2017; undefined(44):113-122
- Campbell WG, Jones BL, Schefter T, Goodman KA, Miften M An evaluation of motion mitigation techniques for pancreatic SBRT. Radiother Oncol. 2017; 1(124):168-173
- Dieterich S, Green O, Booth J SBRT targets that move with respiration. Phys Med. 2018; undefined(56):19-24
- Boda-Heggemann J, Knopf A-C, Simeonova-Chergou A Deep inspiration breath hold-based radiation therapy: a clinical review. Int J Radiat Oncol Biol Phys. 2016; 3(94):478-492
- Shen S, Duan J, Fiveash JB Validation of target volume and position in respiratory gated CT planning and treatment. Med Phys. 2003; 12(30):3196-3205
- Shimizu S, Shirato H, Kagei K Impact of respiratory movement on the computed tomographic images of small lung tumors in three-dimensional (3D) radiotherapy. Int J Radiat Oncol Biol Phys. 2000; 5(46):1127-1133
- Mageras GS, Pevsner A, Yorke ED Measurement of lung tumor motion using respiration-correlated CT. Int J Radiat Oncol Biol Phys. 2004; 3(60):933-941
- Hanley J, Debois MM, Mah D Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation. Int J Radiat Oncol Biol Phys. 1999; 3(45):603-611
- Murphy MJ, Martin D, Whyte R The effectiveness of breath-holding to stabilize lung and pancreas tumors during radiosurgery. Int J Radiat Oncol Biol Phys. 2002; 2(53):475-482
- Lin YH, Ozawa S, Miura H Split-VMAT technique to control the expiratory breath-hold time in liver stereotactic body radiation therapy. Phys Med. 2017; undefined(40):17-23
- Yang W, Fraass BA, Reznik R Adequacy of inhale/exhale breathhold CT based ITV margins and image-guided registration for free-breathing pancreas and liver SBRT. Radiat Oncol. 2014; undefined(9):11-undefined
- Oliver PAK, Yewondwossen M, Summers C Influence of intra- and interfraction motion on planning target volume margin in liver stereotactic body radiation therapy using breath hold. Adv Radiat Oncol. 2021; 1(6):undefined-undefined
- Seppenwoolde Y, Shirato H, Kitamura K Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002; 4(53):822-834
- George R, Chung TD, Vedam SS Audio-visual biofeedback for respiratory-gated radiotherapy: impact of audio instruction and audio-visual biofeedback on respiratory-gated radiotherapy. Int J Radiat Oncol Biol Phys. 2006; 3(65):924-933
- Vedam SS, Keall PJ, Kini VR, Mohan R Determining parameters for respiration-gated radiotherapy. Med Phys. 2001; 10(28):2139-2146
- Goossens S, Senny F, Lee JA, Janssens G, Geets X Assessment of tumor motion reproducibility with audio-visual coaching through successive 4D CT sessions. J Appl Clin Med Phys. 2014; 1(15):4332-undefined
- Lens E, Gurney-Champion OJ, Tekelenburg DR Abdominal organ motion during inhalation and exhalation breath-holds: pancreatic motion at different lung volumes compared. Radiother Oncol. 2016; 2(121):268-275
- Holland AE, Goldfarb JW, Edelman RR Diaphragmatic and cardiac motion during suspended breathing: preliminary experience and implications for breath-hold MR imaging. Radiology. undefined; 2(209):483-489
- Parkes MJ Breath-holding and its breakpoint. Exp Physiol. 2006; undefined(91):1-15
- Parkes MJ, Green S, Kilby W The feasibility, safety and optimization of multiple prolonged breath-holds for radiotherapy. Radiother Oncol. 2019; undefined(141):296-303
- Parkes MJ, Green S, Stevens AM Safely prolonging single breath-holds to >5 min in patients with cancer; feasibility and applications for radiotherapy. Br J Radiol. 2016; 1063(89):undefined-undefined
- Parkes MJ, Green S, Cashmore J, Ghafoor Q, Clutton-Brock T Shortening the preparation time of the single prolonged breath-hold for radiotherapy sessions. Br J Radiol. 2022; 1130(95):undefined-undefined
- Towell V, Gysen KV, Cross S, Kk Low G Efficacy of preoxygenation administration in volunteers, in extending the end-expiration breath-hold duration for application to abdominal radiotherapy. Tech Innov Patient Support Radiat Oncol. 2023; undefined(26):undefined-undefined
- Parkes MJ, Green S, Stevens AM, Clutton-Brock TH Assessing and ensuring patient safety during breath-holding for radiotherapy. Br J Radiol. 2014; 1043(87):undefined-undefined
- Macefield G, Burke D Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain. 1991; undefined(114 (Pt 1B)):527-540
- O’Brien J Absorption atelectasis: incidence and clinical implications. AANA J. 2013; 3(81):205-208
- Kim HI, Min JY, Lee J-R The effect of oxygen concentration on atelectasis formation during induction of general anesthesia in children: a prospective randomized controlled trial. Paediatr Anaesth. 2021; 12(31):1276-1281
- Rothen HU, Sporre B, Engberg G Prevention of atelectasis during general anaesthesia. Lancet. 1995; 8962(345):1387-1391
- Edmark L, Kostova-Aherdan K, Enlund M, Hedenstierna G Optimal oxygen concentration during induction of general anesthesia. Anesthesiology. 2003; 1(98):28-33
- Pingitore A, Gemignani A, Menicucci D Cardiovascular response to acute hypoxemia induced by prolonged breath holding in air. Am J Physiol Heart Circ Physiol. 2008; 1(294):H449-55
- Heusser K, Dzamonja G, Tank J Cardiovascular regulation during apnea in elite divers. Hypertension. 2009; 4(53):719-724
Citation
C S, JB SSF, R J. A Practical Method to Prolong Expiratory Breath Holds for Abdominal Stereotactic Body Radiation Therapy. Appl Radiat Oncol. 2023;(3):34-42.
doi:10.37549/ARO-D-23-00012
September 1, 2023