Malignant Melanotic Nerve Sheath Tumor of the Neck: A Case Report
Affiliations
- 1 School of Medicine, University of Miami Miller, Miami, FL
- 2 David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA
- 3 Mohawk Valley Health System, Utica, NY
- 4 Sylvester Comprehensive Cancer Center, University of Miami Health System, Miami, FL
- 5 University of Texas MD Anderson Cancer Center, Houston, TX
- 6 Massachusetts General Hospital, Boston, MA
- 7 Cleveland Clinic, Taussig Cancer Institute, Cleveland, OH
Malignant melanotic nerve sheath tumors (MMNSTs) are rare, aggressive tumors with significant potential for distant metastasis. This case report describes an atypical presentation of MMNST in a middle-aged patient, arising in the carotid space rather than in the more commonly reported paraspinal regions. The report details clinical presentation, diagnostic evaluation, and the planned course of adjuvant radiation therapy after surgical excision. Comprehensive genomic profiling and multidisciplinary treatment planning were key components of management. Given the rarity of MMNST and its potential for recurrence and metastasis, vigilant, long-term follow-up is essential. This case underscores the importance of recognizing uncommon anatomical presentations that may pose unique diagnostic and therapeutic challenges.
Keywords: nerve sheath neoplasm, malignant, melanotic, adjuvant treatment, case report
Introduction
Melanotic nerve sheath tumors (MMNSTs) are exceptionally rare neoplasms, comprising less than 1% of all nerve sheath tumors, with fewer than 200 cases described in the literature.1 Owing to their rarity, most of the existing knowledge about MMNSTs comes from individual case reports or small case series.2 Management can be particularly challenging owing to the tumors’ potential for aggressive behavior, including local and distant metastasis in over one-third and approximately 44% of cases, respectively.1
Patient History and Surgical Treatment
A middle-aged patient presented to a hospital in September 2023 with complaints of drooping of the right eyelid, neck pain, sore throat, and dull pain in and behind the right ear. The patient had an unremarkable medical history but reported a history of melanoma in the patient’s maternal grandfather. The patient had a 1.25 pack-year smoking history, having smoked 0.25 packs/day for 5 years and last smoking 16 years previous. They reported drinking “socially.”
Right-sided Horner syndrome was confirmed by a positive apraclonidine test. The patient underwent complete resection of the right parapharyngeal space tumor and a right neck dissection. This consisted of transcervical excision of a 4 cm right parapharyngeal space mass seemingly arising from the cervical sympathetic trunk. The mass extended to within 10 mm of the base of the skull and was described as “darker than expected with significant pigment” per surgical note. The mass was resected without complications.
Imaging Findings
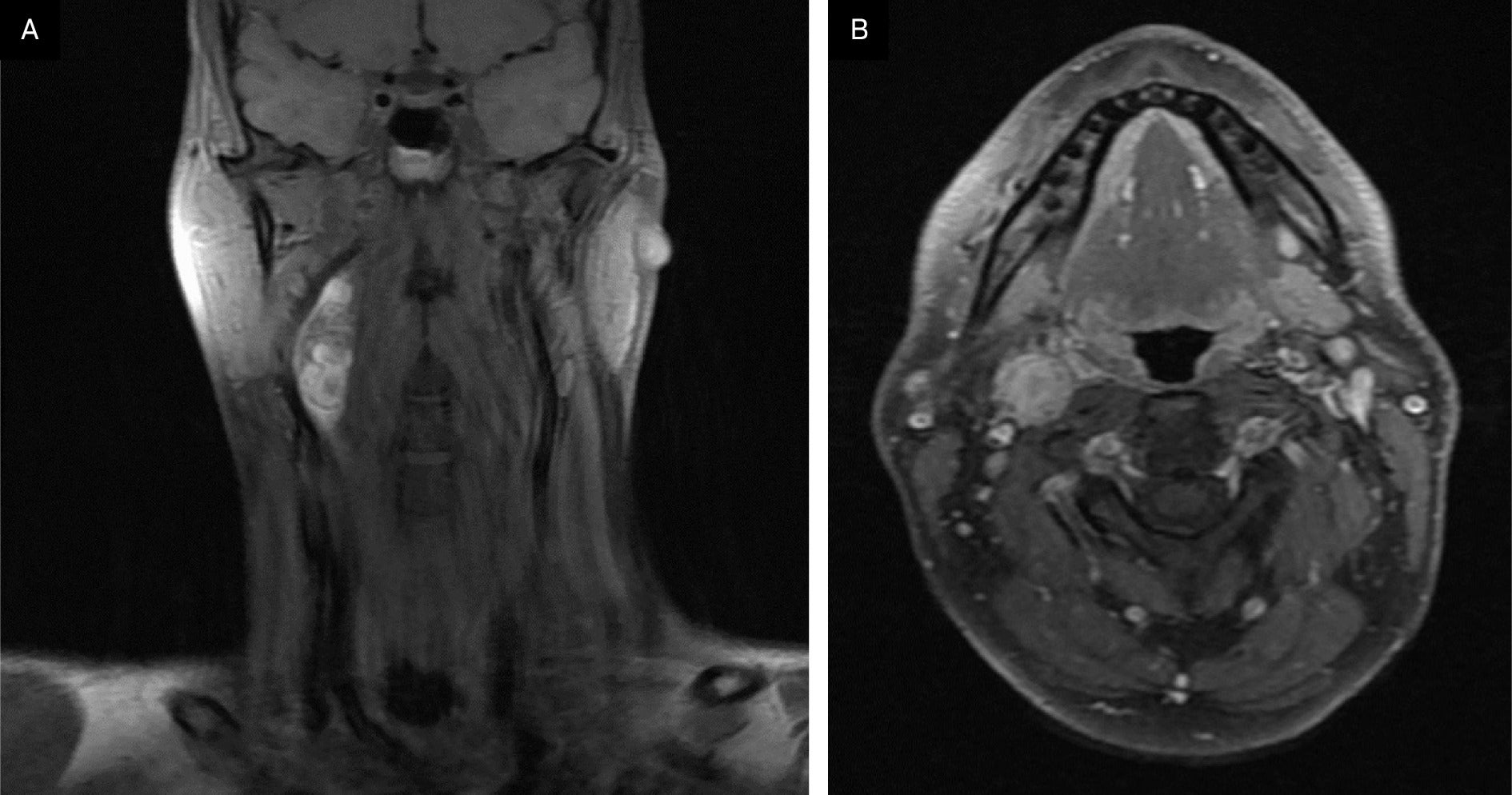
A contrast-enhanced CT neck was significant for a 3.9 × 1.7 × 1.6 cm heterogeneous, partially calcified enhancing lesion in the right carotid space, splaying the internal and external carotid. The radiological differential included a carotid body tumor, with other etiologies like schwannoma or an enlarged lymph node less likely. An MRI demonstrated a 4 cm enhancing lesion in the right carotid space, similarly suspicious for carotid body tumor and, less likely, schwannoma or pathologic lymph node ( Figure 1 ). A chest/abdominopelvic CT scan was negative for thoracic or abdominal paragangliomas and any metastatic disease. A hypermetabolic lesion in the right upper cervical neck was seen on preoperative PET-CT.
Preoperative MRI of the neck showing axial and coronal postcontrast, fat-saturated T1 sequences and demonstrating an enhancing lesion in the right carotid space.
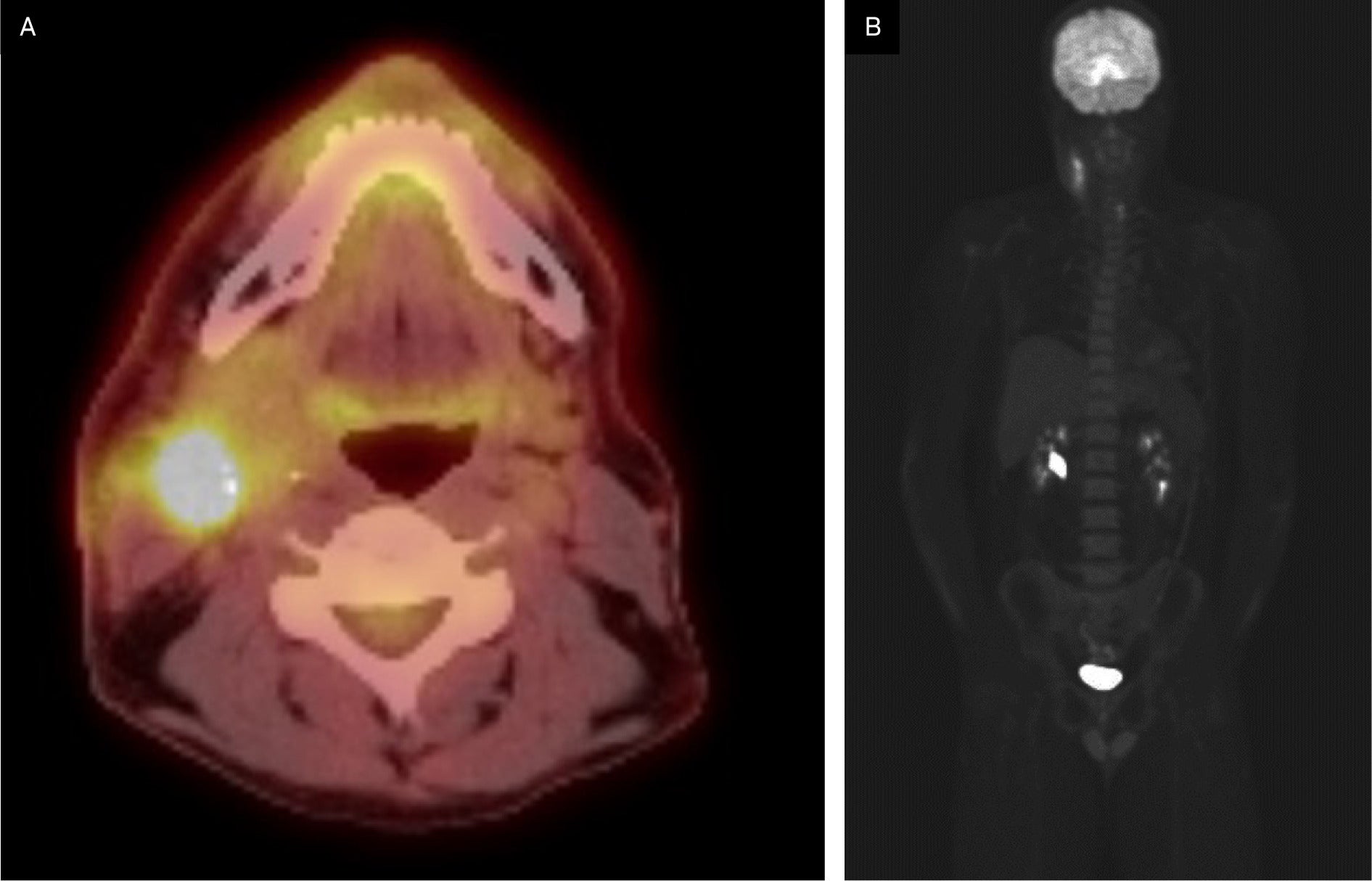
Postoperative PET-CT indicated postsurgical changes in the right upper cervical region with F-18 fluorodeoxyglucose (FDG)-avid, heterogeneous, asymmetric thickening of the anterior aspect of the right sternocleidomastoid muscle and was negative for hypermetabolic lymphadenopathy ( Figure 2 ). There were no findings suspicious for distant metastatic disease.
Preoperative F-18 fluorodeoxyglucose (FDG) PET-CT demonstrating FDG-avid lesions.
Pathology
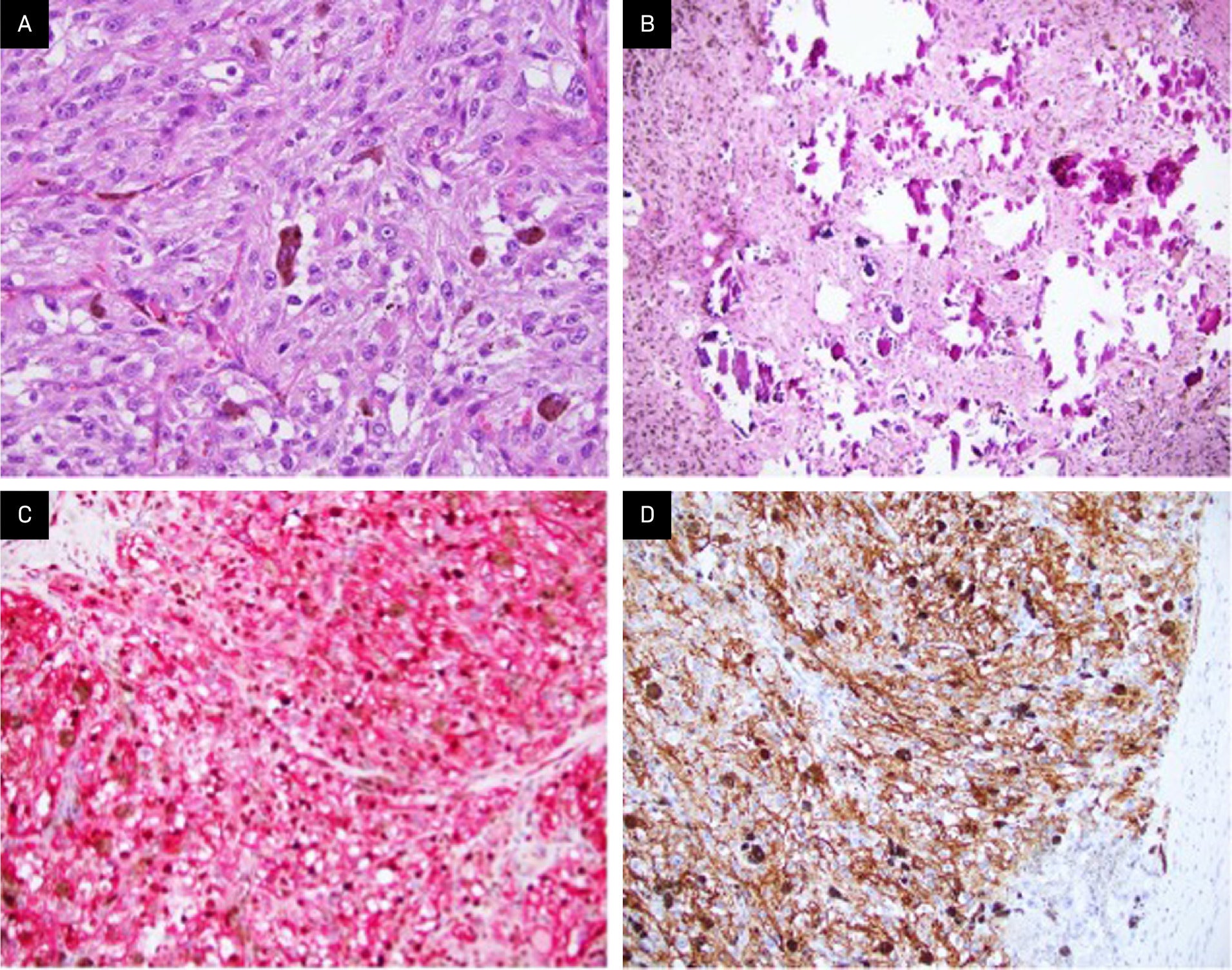
Gross examination of the right parapharyngeal space tumor specimen revealed a 4.0 × 2.0 × 1.4 cm dark tan nodule with a smooth, glistening surface. Serial sectioning demonstrated a well-circumscribed mass with grossly negative margins. Intraoperative frozen-section analysis revealed a pigmented epithelioid neoplasm, with the final diagnosis deferred to permanent sections. Microscopic examination of the permanent sections revealed a well-circumscribed, heavily pigmented neoplasm, arranged in nests and composed of epithelioid cells with round nuclei and prominent nucleoli, with admixed melanophages ( Figure 3A ). Psammoma bodies and areas composed predominantly of melanophages were present ( Figure 3B ). No necrosis, lymphovascular invasion, or perineural invasion was identified. The surgical margins were negative for tumor. A right neck dissection showed 3 lymph nodes negative for malignancy. A combined Melan A/Ki67 immunohistochemical stain of the psammoma bodies was positive for Melan A and showed a Ki67 proliferation index <10% ( Figure 3C ). Immunohistochemistry (IHC) also revealed that the tumor was positive for multiple additional melanocytic lineage markers, including SOX10, S100 protein, MITF, and HMB-45 ( Figure 3D ) but negative for CK8/18 and synaptophysin. IHC for BRAF (VE1 ) was negative in the tumor; thus, there was no indication of an underlying BRAF V600E mutation. PD-L1 expression by IHC was also negative.
Examination shows fascicles of large epithelioid cells with pigmented cytoplasm and prominent nucleoli, as well as admixed melanophages (A, H&E stain; original magnification: ×400). Psammoma bodies typical of this entity are also identified (B, H&E stain; ×200). Immunohistochemistry reveals the tumor is diffusely and strongly positive for melanocytic markers Melan A (C, red chromogen; × 200) and HMB45 (D, × 200), with Ki67 labeling fewer than 10% of tumor nuclei with brown chromogen (C, × 200).
Comprehensive genomic profiling using a hybrid capture-based DNA+RNA sequencing platform (Caris Life Sciences) revealed a PRKAR1A p.S321fs* pathogenic frameshift mutation (RefSeq transcript NM_001276289.1; c.962delC), at 44% variant allele frequency. No genomic alterations in BRAF , CDKN2A, or the TERT promoter were identified. A review of the copy number plot demonstrated monosomies of chromosomes 1, 2, 4, 17, 21q, and 22q. Taken together with the findings of routine histopathologic examination and IHC, this genomic profile, with mutation in PRKAR1A only and monosomies of multiple chromosomes, including 1, 2, and 17, was diagnostic for MMNSTs.
Dedicated constitutional (germline) testing using a DNA and RNA-based platform (Invitae Multi-Cancer+RNA Panel) was performed on whole blood and was negative for all alterations, including in PRKAR1A . This finding supports that the above PRKAR1A mutation identified in tumor tissue is somatic in origin.
Discussion
MMNSTs, previously termed melanotic schwannomas, are uncommon tumors classified by the presence of melanin-producing Schwann cells.3 These entities are known for their aggressive nature and rarity as they make up <1% of all nerve sheath tumors.2 They typically originate in the spine or paraspinal soft tissue, although this particular case report presents one within the carotid space.4 Psammomatous MMNSTs account for approximately half of all MMNSTs, and approximately half of these tumors are associated with the Carney complex, a disorder characterized by skin pigmentation, endocrine abnormalities, and increased risk of pituitary adenomas, testicular tumors, and cardiac myxomas.5 This disorder is often associated with mutations in the PRKAR1A gene. However, in this case, the patient does not have a history of the Carney complex, and dedicated germline testing was negative for PRKAR1A mutation.
In the limited number of MMNST cases reported, patients commonly presented with neuropathic symptoms such as pain and paresthesia, similar to the findings in this case.6
Key imaging features include enhancement at CT and FDG-avidity at PET-CT. It has also been noted that MMNSTs typically grow along a spinal nerve root with a unique “dumbbell” configuration, though this is a nonspecific finding, as many types of tumors assume this configuration if they contain intradural and extradural components.7 - 9 MRI is useful in distinguishing them from other tumors, as MMNSTs have intrinsic T1 hyperintensity ( Figure 1 ), while schwannomas and neurofibromas tend to be hypointense on T1 and hyperintense on T2.10
MMNSTs are also characterized microscopically by spindle and epithelioid cells arranged in interlacing fascicles, with marked accumulation of melanin in neoplastic cells and admixed melanophages. Psammoma bodies are also typical.3 Protein kinase A regulatory subunit-α ( PRKAR1A ) is genomically inactivated in MMNST. Monosomies of chromosomes 1, 2, and 17 are also characteristic.11 This entity is distinguished from other heavily pigmented melanocytic neoplasms, including pigmented epithelioid melanocytoma and melanoma, by the presence of these specific monosomies and a lack of additional genomic alterations ( TERT promoter, CDKN2A gene mutations, etc) encountered in melanoma.12
Complete resection is the primary curative treatment, with prior reports suggesting adjuvant radiation for larger or more aggressive lesions.1, 13 However, studies have found that 35% of patients experience local recurrences, suggesting that adjuvant radiation should be considered more broadly, particularly given the sensitive locations in which these neoplasms often arise that prohibit meaningful salvage operations. MMNSTs also have notable malignant potential; in one study, nearly half of the patients developed metastases.1 Although MMNSTs are histologically and clinically distinct from malignant peripheral nerve sheath tumors (MPNSTs), treatment strategies are often extrapolated from the MPNST literature owing to the rarity of MMNSTs. Targeted therapies such as oncolytic herpes simplex virus have shown promise in preclinical models of MPNSTs.14 Similarly, multimodal treatments such as preoperative chemotherapy, surgery, and postoperative radiation therapy (RT) have led to extended remission in some cases of MPNSTs.15 Further research is needed to identify effective targeted therapies and treatment strategies for MMNSTs.16 As noted in Table 1 , data documenting a strong association between adjuvant treatment and outcomes after surgical treatment of MMNSTs are lacking.
Malignant Melanotic Nerve Sheath Tumor Case Reports and Treatment Outcomes
| N | Age/Gender | Location of Tumor | Type of Surgery (R0, R1, R2) | Adjuvant Treatment | Radiation Dose if Received | Outcome (30 mo NED, etc) |
|---|---|---|---|---|---|---|
| 1 | 52 y/male17 | Parotid gland | R1 | None | None | No recurrence |
| 1 | 21 y/female18 | Left foraminal L5/S1 | R0 | None | None | Recurred (i.e., locally at L5/S1) and metastasized (ie, leptomeningeal spread) throughout the neuroaxis within 4 postoperative months |
| 1 | 45 y/female19 | C6 nerve root, with both intradural and extradural components | R0 | RT and then combination immunotherapy with nivolumab and ipilimumab | Not specified | Recurrence and widespread metastasis with death at 15 mo after diagnosis |
| 1 | 59 y/female20 | Left paraaortic area | R0 | The tumor was resected en bloc by laparoscopic surgery and subsequent adjuvant RT | Not specified | No evidence of recurrent or metastatic disease 11 mo post- RT |
| 1 | 18 y/female21 | S1 nerve root | R0 | Adjuvant stereotactic radiosurgery | 40 Gy in 5 fractions, prescribed to 84% isodose line, with 25 Gy to bony spinal canal | No evidence of recurrent or metastatic disease after 2.5 y |
| 2 | 58 y/female; 72 y/male6 | T11-T12; T11 | R0; R1 | Case 1: complete resection with no adjuvant treatment Case 2: incomplete resection due to severe adhesions and bleeding, continued annual MRI, abdominal CT, and CXR without distant metastasis |
None | None in both cases |
| 2 | 35 y/male; 50 y/female2 | Lumbar spinal L1/2 to L3/4 and cervical spinal at C6 | R2, R1 | Case 1: L2-L3 laminectomy and adjuvant RT Case 2: C5-7 laminectomy with partial excision of the lesion and adjuvant RT |
Case 1: RT; 50.4 Gy in 28 fractions Case 2: 50.4 Gy followed by 5.4 Gy boost |
Case 1: no recurrence; currently on follow-up 6 mo post RT Case 2: therapy ongoing |
Abbreviation: R0, resection with cure or complete remission; R1, resection with microscopic residual tumor; R2, resection with macroscopic residual tumor; RT, radiation therapy.
Adjuvant Treatment
After consultation with the patient, adjuvant RT was recommended owing to the locally aggressive nature of this tumor. The radiation dose required for optimal disease control remains uncertain; multiple experts recommend 60-70 Gy in standard and slightly hypofractionated regimens, consistent with that for aggressive soft tissue sarcomas or MPNSTs of the head and neck with negative surgical margins.22, 23 In this case, a compromise was reached among radiation oncologists to use an integrated boost where the postop bed/high-risk area was treated to 63 Gy in 2.1 Gy fractions; the intermediate-risk area was treated with 60 Gy in 2 Gy fractions to a radial margin of 0.5 cm on the postop bed, cropped back from bone, air, and 1.5 cm superior/inferior margin on the postop bed for concern about tracking along the vessels and nerves. The low-risk area was treated with 54 Gy in 1.8 Gy fractions to level 2b and the rest of level 3, to complete contouring of the involved LN basins surrounding the tumor bed and to add small margins on the postop bed. The regional lymph nodes were included in the low-dose area because of the possibility of aggressive local behavior and the propensity of head and neck cancers to metastasize to the regional neck nodes.
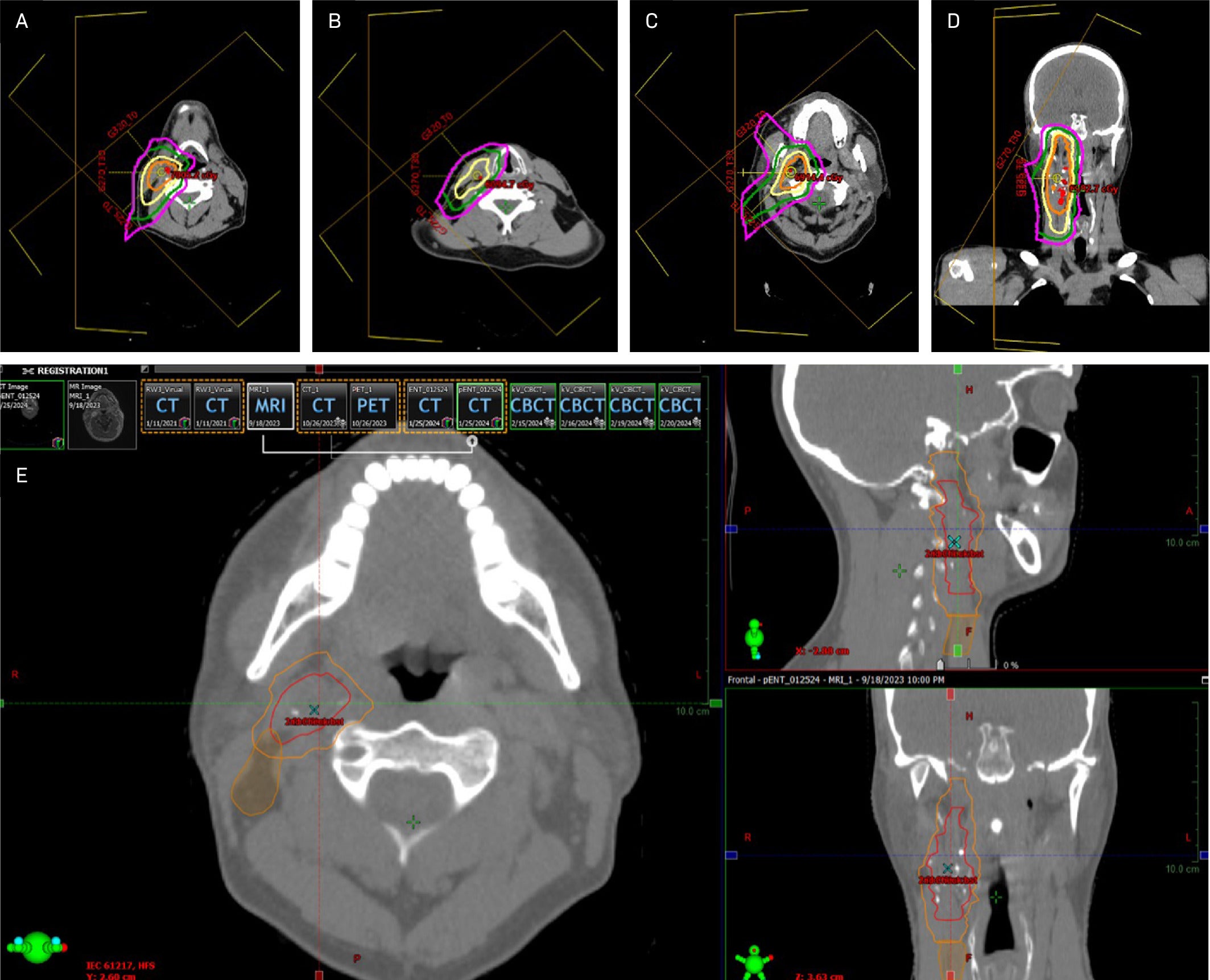
The patient was simulated supine with a long Aquaplast mask. Given their young age and planned ipsilateral treatment, intensity-modulated proton therapy delivered in Gy (RBE) was recommended to minimize radiation dose to nearby and contralateral healthy organs based on improved radiation dosimetry compared with IMRT. Image guidance with daily cone beam CT was utilized to minimize interfraction variability. No planning target volumes were added because the patient was treated with proton therapy. Slices from the primary RT plan are shown in Figure 4 . The patient did not miss any treatments and tolerated the expected side effects of treatment, including grade 1 dermatitis of the right neck and right cheek, along with hair loss in the treatment field. However, the patient experienced no fatigue, changes in taste or saliva, or mucositis.
Axial and coronal images of the final proton therapy plan. Axial slices (clockwise from top left) correspond to the superior field (A, B), mid-field (C, D), and inferior field (E). The patient was treated with 3 anterolateral proton beams using pencil-beam scattering. Isodose lines represent (from innermost to outermost): 63 Gy (orange), 54 Gy (yellow), 31.5 Gy (green), and 15.75 Gy (fuchsia).
Post-Treatment Follow-Up
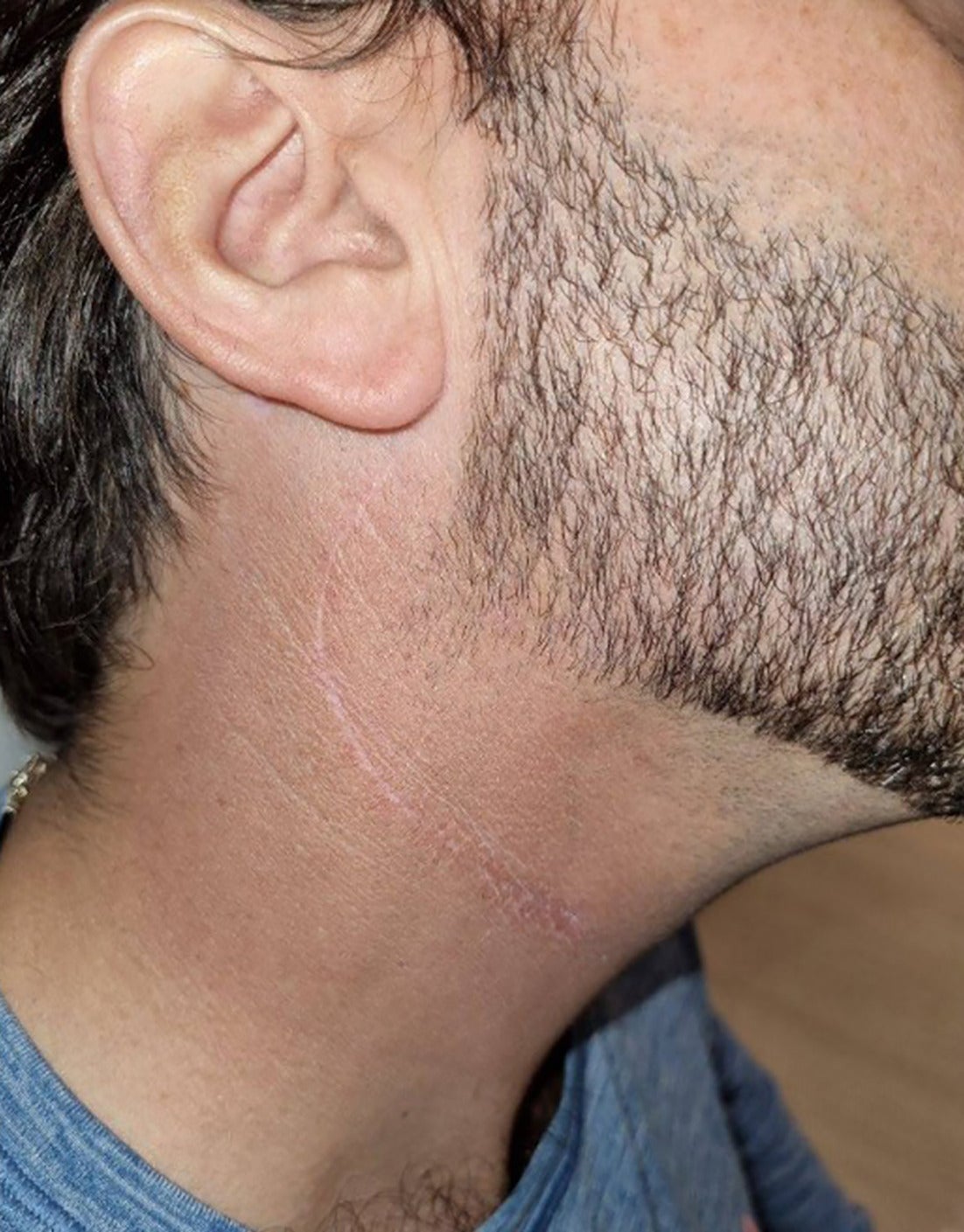
At evaluation 3 months postradiation, the patient’s skin showed no residual reaction other than mild hyperpigmentation. Beard on the cheek, but not on the neck, grew back ( Figure 5 ).
Clinical photograph of the right neck 6 mo post-treatment.
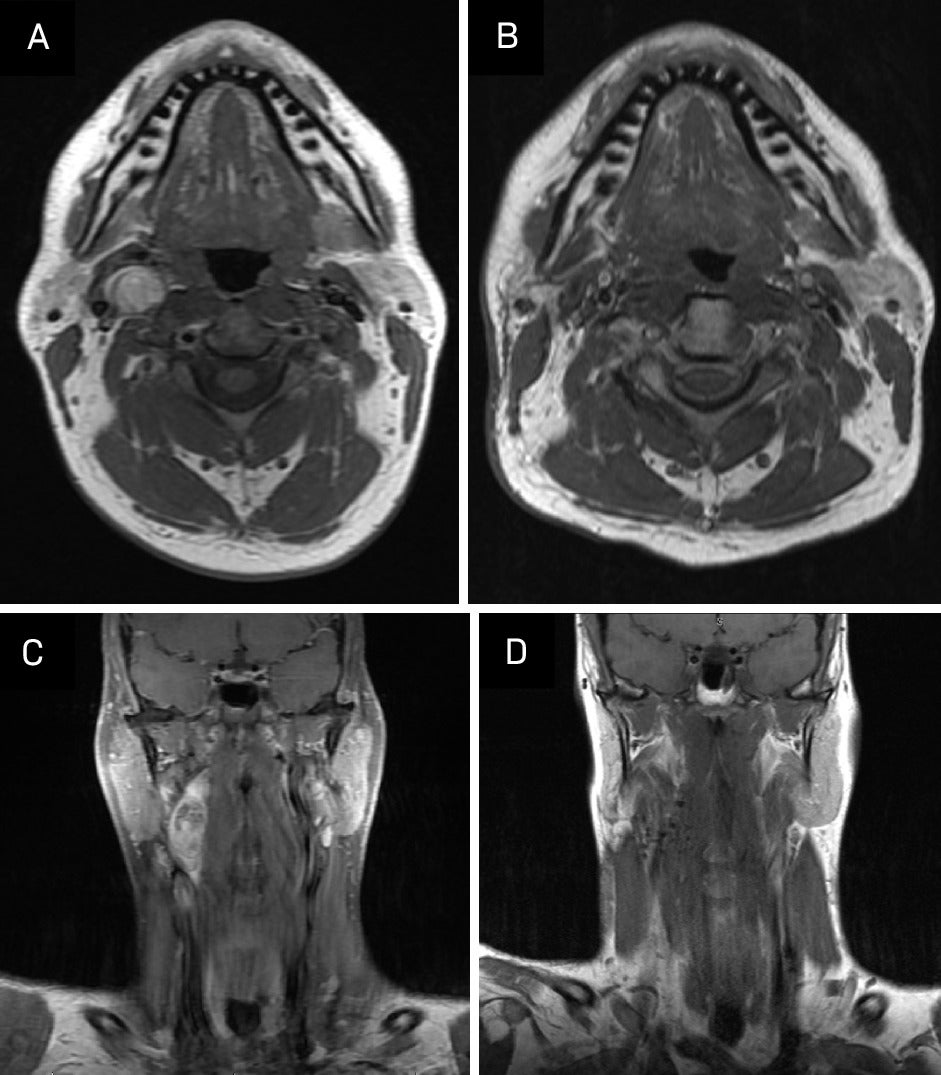
MRI 3 months post-treatment demonstrated no evidence of local or regional recurrence. Imaging showed increased signal on STIR and some enhancement in the right aspect of the neck, including the right aspect of the oropharynx and supraglottic larynx, presumably postradiation effects. There was also increased T2 signal and enhancement in the right submandibular gland, which was also compatible with postradiation effects ( Figure 6 ). We plan to repeat MRI every 6 months for 2 years and then yearly for 5 years. Routine clinical follow-up will be scheduled after every imaging study.
Comparison of pretreatment and post-treatment imaging. (A, C) Pretreatment: T1-weighted fast spin echo (FSE), noncontrast. (B, D) Post-treatment: T1-weighted turbo spin echo (TSE), noncontrast.
Conclusion
Managing MMNSTs remains challenging owing to their rarity and aggressive nature, and presenting these cases to multidisciplinary tumor boards is imperative. Data to guide treatment recommendations are lacking; our case highlights the need for further research to improve understanding of, develop data-driven treatment recommendations for, and improve long-term patient outcomes in this rare malignancy.
References
Citation
Ship H, Shehadeh S, Montoya C, Freedman L, Jin W, Rich B, Bishop AJ, MacDonald S, Campbell SR, Milikowski C, Montgomery E, Williams EA, Arnold D, Samuels SE. Malignant Melanotic Nerve Sheath Tumor of the Neck: A Case Report. Appl Radiat Oncol. 2025;(2):1 - 7.
doi:10.37549/ARO-D-25-00007
June 1, 2025