Disparities in Colorectal Cancer Outcomes Among Young Adults and African Americans in the United States
Images
SA-CME credits are available for this article here.
Disparities in colorectal cancer outcomes for young adults (YA) and African Americans (AA) have been long acknowledged within the medical community. Colorectal cancer (CRC) is the third most common cancer in men and women, and a leading cause of cancer-related death throughout the United States. In 2020, it is projected that 147,950 individuals will be newly diagnosed with CRC, with 104,610 of these cases presenting as colon cancer (CC) and 43,340 as rectal cancer (RC).1 Presentation with advanced-stage disease attributable to delayed diagnosis leads to less favorable outcomes; thus, early detection is considered a means to decrease deaths associated with CRC.2 Additionally, modifiable risk factors like physical inactivity, smoking, obesity and poor diet are responsible for a proportion of the cancer recurrences and deaths in patients diagnosed with CRC.3
While the incidence of CRC in people over age 50 has declined from 2001 to 2012, the incidence rates of CRC in YAs (< 50 years) have continued to increase since the mid-1990s.4-6 By the turn of the century, most notable changes in this uptrend were highlighted in the youngest age group (20 to 35 years).4-6 Analyses of the most recent data years (2012 to 2016) have found that incidence rates increased by 2.2% and 1.1% annually in individuals younger than 50 and 50–64, respectively.7 This data is in contrast to adults aged 65 and older, whose incidence rates have decreased by 3.3% annually.7 These rising incidences will account for an estimated 12% of the total projected 147,950 cases in 2020 to be diagnosed in patients younger than 50.1 In terms of mortality rates, YAs diagnosed with CRC will contribute to 7% of CRC-related deaths this year.1
Age group differences are not the only disparity observed when looking at outcomes in CRC treatment and survival. The American Cancer Society (ACS) reports that during the most recent data-gathering period (2012 to 2016) CRC incidence rates in AA were 20% higher than those in non-Hispanic Whites, and mortality rates were double the incidence, 40% in AA compared to non-Hispanic Whites.7 In this article, we review current literature continuing to highlight persistent disparities in the diagnosis, treatment and survival outcomes in CRC in YAs and AAs.
Screening Recommendations
The U.S. Preventive Services Task Force (USPSTF) and Centers for Disease Control and Prevention (CDC) recommend CRC screening for average-risk people aged 50 to 75 years. Average risk includes adults who do not have a personal or family history of CRC or certain types of polyps, no history of inflammatory bowel diseases (ulcerative colitis or Crohn’s disease), no confirmed or suspected hereditary CRC syndrome, and no personal history of previous radiation therapy in the abdomen or pelvis.8 Screening can include: fecal occult blood test (FOBT), fecal immunochemical test (FIT), a combination of stool DNA and FIT test (FIT-DNA), computed tomography (CT) colonography, flexible sigmoidoscopy, or colonoscopy.9 From 2009 to 2015, the CDC implemented the first public health program focused solely on increasing the use of CRC screening tests at a population-based level, the Colorectal Cancer Control Program (CRCCP).9 However, 2016 data continued to highlight that only 67% of adults ages 50 to 75 were up to date with screening, and 26% of this age cohort had never been screened.9
The U.S. Multi-Society Task Force (MSTF) recommends that CRC screening should begin at age 50 in average-risk persons, except AAs in whom limited evidence supports screening at age 45 years.10 The MSTF-recommended tests are ranked into 3 successive tiers based on performance features, costs and practical considerations. Tier 1: colonoscopy every 10 years and annual FIT. Tier 2: CT colonography every 5 years, FIT-fecal DNA every 3 years and flexible sigmoidoscopy every 5-10 years. Tier 3: capsule colonoscopy every 5 years.10
The American Cancer Society (ACS) offers a qualified recommendation for patients aged 45 and a strong recommendation for patients aged 50 years, that adults with an average risk of CRC should undergo regular screening. CRC screening options include annual FIT, annual guaiac-based fecal occult blood test, multitarget stool DNA test every 3 years, colonoscopy every 10 years, CT colonography every 5 years, and flexible sigmoidoscopy every 5 years.8 With acknowledgement of variability in test type availability, effectiveness, and patient burden, the ACS endorses that screening with any of these methods is associated with a significant reduction in CRC incidence through early detection and removal of polyps and other precancerous lesions.8 By involving patients in decision-making, the ACS hopes to increase the likelihood of long-term adherence.8
Age Disparity
Since 2000, the increasing rates of CRC diagnoses have been documented in patients younger than 50, with the greatest increase in patients 20 to 35 years.6 With a growing incidence of 2.2% annually for this age group, models projected by Bailey et al expect the incidence rates of CRC in YA to nearly double by 2030.6,11 Clinicopathologic and molecular features of CRC in YA differ from those who develop CRC after age 50. Patients < 50 more often present with advanced disease characterized by tumors with aggressive histologic features and synchronous metastases.12-14 Additionally, primary tumors in YA are normally localized in the rectum and distal (left) colon.14 Colorectal cancers are classified by major histological subtypes: adenocarcinoma, mucinous adenocarcinomas, signet-ring cell carcinomas and several additional rare subtypes. In patients > 50 years, adenomatous polyps and adenomatous polyposis coli (APC) mutations are more common, while in young patients (18 to 29 years), signet-ring cell cancer is more prevalent.14,15 In the realm of all CRC cancer types, signet-ring cell carcinoma is rare and associated with poor prognostic factors.16
Inherited syndromes associated with abnormal genes passing from generation to generation are known to increase the likelihood of certain cancers in YAs. Polyposis and nonpolyposis syndromes are inherited syndromes recognized to predispose YAs to CRC. The most common polyposis syndrome, familial adenomatous polyposis (FAP), is an autosomal dominant syndrome affecting the APC tumor suppressor gene.17 Loss of function in APC causes polyps to develop in hundreds to thousands throughout the colon and rectum. Polyp development typically begins at ages 20 to 30, and without prophylactic colectomy, the lifetime risk of developing CRC approaches 100%.17 Lynch syndrome (LS) is the most common nonpolyposis hereditary cancer syndrome associated with CRC and endometrial cancer predominantly.17 LS is caused by an autosomal dominant heterozygous germline mutation in the DNA mismatch repair genes. Polyps developing in patients with LS progress to carcinoma often faster than sporadic cases, and these patients have a CRC lifetime risk reaching 70%, with 40% of LS patients diagnosed with CRC before age 40.17 Microsatellite instability (MSI) phenotype is present in many cancers, but has been extensively characterized in CRC and is a diagnostic feature of LS.18,19 MSIs result from a germline mutation in mismatch repair (MMR) genes MLH1, MLH2, MLH6, PMS2 or a germline deletion in epithelial cell adhesion molecule (EPCAM).17,18 A retrospective review of > 36,000 CRC patients found that patients with recognized hereditary syndromes were more likely to have a high level of microsatellites and to be diagnosed under age 50.12 In conjunction with family history, microsatellite identification is a first step when diagnosis of LS is suspected.19 Guidelines supported by the National Comprehensive Cancer Network recommend universal testing for all patients with newly diagnosed CRC to identify deficient MMR or MSI to determine LS association.20 While the outlook for LS-associated CRC may be promising, heritable syndromes (including LS and FAP among others) only account for roughly 35% of CRC cases in YAs, leaving a greater proportion of colorectal malignancies in this age group presenting with sporadic cases.15,17 Therefore, risk factors associated with sporadic CRC in the YA population warrant further investigation.
Evaluation of recent studies conducted by Burnett-Hartman et al suggests that stage-specific survival among YAs diagnosed with CRC is equivalent to, or better than, survival outcomes for patients > 50 years.14 When evaluating the treatment and outcomes of young CRC patients in community-based health care systems, this study found that a majority (83%) of YAs with CRC receive surgery at comparable rates to patients > 50. Additionally, YAs were more likely to receive systemic therapy within 6 months of diagnosis as compared to counterparts > 50 years.14 Risk of all-cause mortality and mortality due to CRC was lower in early onset patients than older patients (all cause = HR O.66 CI 0.58-0.75; CRC specific = HR 0.66 CI 0.56-0.79).14 These findings align with another population-based study using the Surveillance, Epidemiology, and End Results (SEER) database to determine treatment plans for average-risk patients who presented with CRC before the recommended screening age (37,847 patients, 14.7% of cohort).21 They found that younger patients with distant metastases were more likely to still receive surgical therapy for the primary tumor followed by radiation therapy.21 Aggressive treatment methods in patients < 50 have supported better overall disease-specific survival. Adjusted 5-year cancer-specific survival for patients < 50 was better for localized (95.1% vs 91.9% P < 0.001), regional (76% vs 70.1% P < 0.001), and distant disease (21.3% vs 14.1% P < 0.001), despite a larger percentage of YA presenting with more advanced disease.14,21
Racial Disparity
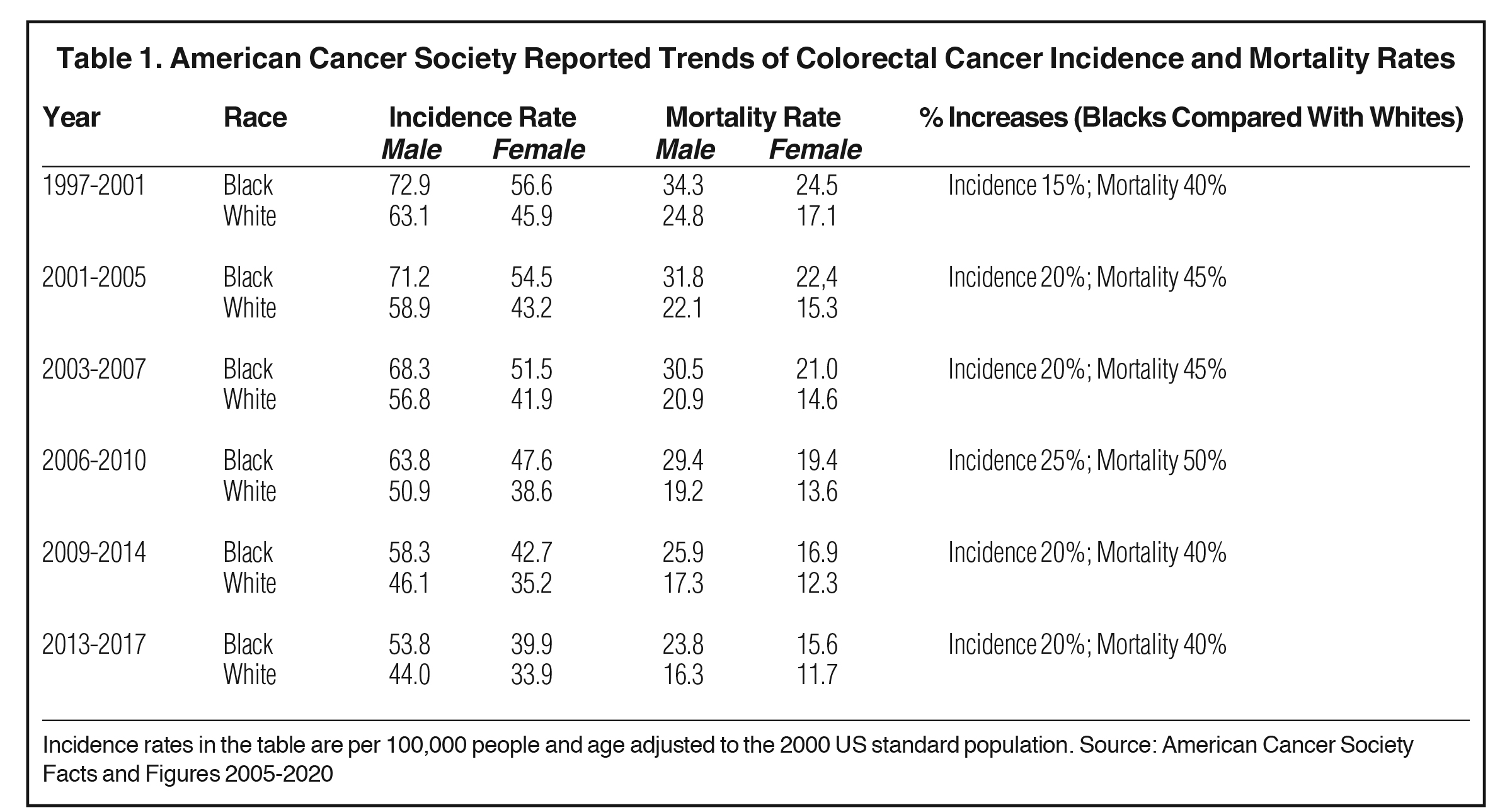
Historically, AAs have had higher incidence rates of CRC and poorer survival outcomes than those of White counterparts.8,22 According to the ACS, in 2005 the incidence rate of CRC in AAs was 15% higher than in Whites, and mortality rate for AAs was 40% higher than in Whites7 — trends that have continued (Table 1). Most recent data reported in 2020 show the relative difference in CRC incidence rates between Whites and AAs is now 20% higher for AAs.7 Relative differences in mortality rates have remained at 40%.7 These growing and persistent disparities are problematic because CRC is a treatable cancer when detected as precancerous or localized malignant lesions.23 In 2010, racial disparities were thought to be explained by differences in socioeconomic status between AAs and Whites.22 Yet today, many intrinsic and extrinsic factors are recognized contributors to the disparate incidence rates and outcomes of CRC in AAs. Intrinsic factors may include comorbidities, lifestyle choices, medical mistrust, and tumor characteristics. Extrinsic factors may include poverty, insurance status, accessibility to quality health care (medical treatment, surgical treatment) and implicit bias among physicians and established US health care systems.24–29 Each factor contributes to lower rates of CRC detection and inferior cancer-specific outcomes in AAs as compared to Whites. As mentioned, overall CRC screening rates for the entire population are estimated to be 60% to 70%.30 A recent prospective cohort study of 47,596 adults > 50 years looked at the use of CRC screening among AAs.30 Baseline colonoscopy rates were significantly lower among AAs (67.3% vs 75.5%) than Whites; meanwhile, sigmoidoscopy usage rates were similar across the racial groups.30 For patients who had undergone colonoscopy or sigmoidoscopy at the time of baseline screening, a 46% decreased risk of CRC was detected.30
A systematic review from 2011 identifying gaps in CRC screening among AAs concluded that three levels of modifiable barriers can potentially improve screening rates. These include issues at the level of patient barriers, provider barriers, and systemic barriers.24,31 Patient barriers included psychological factors (fear) and low health literacy regarding CRC risk and perceived susceptibility.31 Provider barriers included confusion about age recommendations, low acknowledgement of patient barriers and lack of provider recommendation for colonoscopy (the most frequently reported provider barrier to CRC screening in AA of those listed).31 Systemic barriers included costs, insurance coverage, fewer specialist referrals and limited primary care visits.31 From 2008 to 2016, implementation of the Affordable Care Act (ACA) and expansions in Medicaid increased access to CRC screening.32 During this period, screening in Whites increased by 0.76% (P < 0.001) while for AAs, the rate increased significantly by 1.14% (P < 0.001) per year from 2008 to 2014 and then remained stagnant from 2014 to 2016.32 While these numbers are encouraging, the change did not surpass the previously reported disparity in screening, and screening numbers for Whites continued to exceed AAs and all other racial groups. Overall they found that the absolute difference in screening rates for Whites became smaller, while for Blacks, the disparity in screening slightly increased (3.3% to 4.0%).32
Another barrier to equitable health outcomes identified by a National Cancer Institute study was poor recruitment, enrollment and retention levels of AA patients in clinical trials.33 The study noted that the numbers of AAs enrolled and retained in clinical trials were not representative of the minority population numbers across the US,33 thus decreasing generalizability. Not surprisingly, at a study center where special recruitment efforts (including additional recruitment costs) for AAs were implemented, levels of minority representation for this study equaled or exceeded the levels of the catchment population.33 In order to engage AA participants, two studies concluded that the most effective mechanism was to address cultural factors.24,33 This was achieved by providing accurate information to help overcome a sense of mistrust about clinical trials, promoting community outreach via trusted organizations, and by having Black staff and investigators available to interact with participants.33
As highlighted by the ACA, CRC outcomes for AA patients are worse than those of Whites.1 One study of 199,098 CRC patients from the National Cancer Database (NCDB) from 2004-2012 compared overall 5-year survival across non-Hispanic Black and non-Hispanic White patients.28 They found that AA patients were more likely to be diagnosed at a younger age (28.1% vs 26.2%), have right-sided colon cancer (33.3% vs 24.1%), and present with stage IV disease (27.6% vs 22.6%).28 Upon matching by insurance status, the proportional difference in AA and Whites presenting with metastatic disease decreased by 2.2%.28 Unmatched 5-year survival outcomes for this cohort showed a 9.2% difference (95% CI, 57.3% [56.6 to 57.9] for AAs and 66.5% [66.3 to 66.8] for Whites).28 After matching by insurance status, the difference decreased to 4.9%.28 Insurance also played an important role in CRC outcomes based on surgical intervention. While patients across all races undergoing surgical treatment were equally likely to receive colorectal resection by laparoscopic surgical technique, AAs as compared to White patients were more likely to have postoperative complications (OR 1.23, 95% CI 1.17-1.29), including bleeding, cardiac failure, renal failure and respiratory failure.29 When the data was stratified by insurance type, patients with private insurance were more likely to have laparoscopic procedures as compared to all other insurance types.29 Patients with Medicare or Medicaid were more likely to have postoperative complications (OR 1.30, 95% CI 1.24 to 1.37, OR 1.40, 95% CI 1.31 to 1.50, respectively).29
Physician bias is an emerging field of study without clear recommended methodology. Despite the conceptual nature of bias, disparate health outcomes due to implicit biases are tangible and therefore this factor must be addressed. A systematic review evaluating 27 articles found evidence for implicit bias among physicians and nurses, manifested at levels to a similar degree as the general population.27 This is concerning as variability in rates of CRC screening and treatment recommendations for AAs are likely influenced by this phenomenon.
Racial and Age Disparity
In addition to existing disparities for CRC incidence and outcomes for age and race independently, differences in outcomes are reported between AA and White patients in the growing cohort of YA patients. A population-based study utilizing SEER data from 2004 to 2011 found that in all patients under age 50, 19% were diagnosed in AA patients, compared to 16% in Whites.34 Furthermore, young AA patients continue to be diagnosed at later tumor stages and have poorer outcomes.34,35 Since differences in screening cannot be attributed to an age group < 45 years, differences in tumor biology have been considered as a potential association with poorer outcomes for this race group.35 While AAs have lower median survival for proximal, distal and rectal disease,34 primary tumor location differs for AA and White patients. Cancer arising in the colon is found in a higher proportion of AA patients (71.6%) than White (58.2%), and AA patients are more likely to develop proximal CC, which is an independent risk factor for poor outcomes across all ethnic and racial groups.34 The median time from diagnosis to treatment, surgery and chemotherapy are comparable across race groups, but AAs had the lowest frequency of radiation therapy use.35 Despite comparable treatment efforts, AAs have significantly lower median and 5-year survival rates (Blacks 58.8% vs Non-Hispanic Whites 66.9%, P < 0.001).35
Conclusion
Disparities in CRC detection, diagnosis and survival outcomes continue to persist on the basis of age and race. The growing issue of YAs being diagnosed with CRC raises concern as only a fraction of these cases can be attributed to hereditary syndromes with a predictable clinical sequalae. Despite the predicted surge of CRC cases in YAs by 2030, there has been no updated screening guidance or qualified recommendation to address the growing cohort of CRC patients < 45 years old. The choice to not screen adults under age 45 is based on a lack of supporting evidence that screening average-age-risk individuals < 50 years will translate to increased early detection of CRC or increased patient survival.36 Similarly, without full understanding of CRC biology in young adults, clear benefit vs increased risk in early screening cannot be guaranteed.37 Fortunately, YAs diagnosed with CRC can often withstand more aggressive treatment regimens, and reported staged-based survival outcomes are comparable to older counterparts.
These considerations further support the importance of heightened awareness among both physicians and the general population about the CRC uptrend in YAs. Beginning education early with medical students and positioning continued awareness toward primary health personnel may help to improve this emerging epidemiological trend.38
To alleviate the burden of CRC on AA communities, changes are needed to narrow the gap in access, prevention and treatment. Despite targeted efforts to promote screening and engagement of AA populations in research trials, comparable utilization rates to Whites have not yet been achieved. Culturally based interventions and health policy changes24,33 have proven useful; however, these are only addressing one element of the greater issue. Since tumor characteristics and genetics cannot solely account for the disparities, structural barriers such as insurance and access to care play a role in the treatment of AAs and overall patient outcomes. Furthermore, investigation into the impact of physician bias on patient prevention counseling, time to treatment, and recommended therapy options is warranted.
References
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164.
- Connell LC, Mota JM, Braghiroli MI, Hoff PM. The rising incidence of younger patients with colorectal cancer: questions about screening, biology, and treatment. Curr Treat Options Oncol. 2017;18(4):23.
- Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol. 2015;33(16):1825-1834.
- Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970-2014. JAMA. 2017;318(6): 572-574.
- Henley SJ, Ward EM, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2020;126(10):2225-2249.
- Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13(2):109-131.
- American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022. Accessed September 16, 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250-281.
- Joseph DA, DeGroff A. The CDC Colorectal Cancer Control Program, 2009-2015. Prev Chronic Dis. 2019;16:E159.
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112(7):1016-1030.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150(1):17-22.
- Weinberg BA, Marshall JL, Salem ME. The growing challenge of young adults with colorectal cancer. Oncology (Williston Park). 2017;31(5):381-389.
- Khan A, Ituarte PHG, Raoof M, et al. Disparate and alarming impact of gastrointestinal cancers in young adult patients. Ann Surg Oncol. 2020.
- Burnett-Hartman AN, Powers JD, Chubak J, et al. Treatment patterns and survival differ between early-onset and late-onset colorectal cancer patients: the patient outcomes to advance learning network. Cancer Causes Control. 2019;30(7):747-755.
- Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019;125(12):2002-2010.
- Yang LL, Wang M, He P. Clinicopathological characteristics and survival in colorectal signet ring cell carcinoma: a population-based study. Sci Rep. 2020;10(1):10460.
- Levine O, Zbuk K. Colorectal cancer in adolescents and young adults: Defining a growing threat. Pediatr Blood Cancer. 2019;66(11):e27941.
- Sinicrope FA. Lynch syndrome-associated colorectal cancer. N Engl J Med. 2018;379(8):764-773.
- Baudrin LG, Deleuze JF, How-Kit A. Molecular and computational methods for the detection of microsatellite instability in cancer. Front Oncol. 2018;8:621.
- Provenzale D, Gupta S, Ahnen DJ, et al. Genetic/Familial High-Risk Assessment: Colorectal Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(8):1010-1030.
- Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM, Hendren S. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. 2016;122(6):929-934.
- White A, Vernon SW, Franzini L, Du XL. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010;116(19):4622-4631.
- Ahmed M. Colon cancer: a clinician’s perspective in 2019. Gastroenterology Res. 2020;13(1):1-10.
- Roy S, Dickey S, Wang HL, et al. Systematic review of interventions to increase stool blood colorectal cancer screening in African Americans. J Community Health. 2020:1-13.
- Adams LB, Richmond J, Corbie-Smith G, Powell W. Medical mistrust and colorectal cancer screening among African Americans. J Community Health. 2017;42(5):1044-1061.
- Delisle M, Singh S, Howard J, Panda N, Weppler AM, Wang Y. Refusal of colorectal cancer surgery in the United States: predictors and associated cancer-specific mortality in a Surveillance, Epidemiology, and End Results (SEER) cohort. Surg Open Sci. 2020;2(4):12-18.
- FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19.
- Sineshaw HM, Ng K, Flanders WD, Brawley OW, Jemal A. Factors that contribute to differences in survival of black vs white patients with colorectal cancer. Gastroenterology. 2018;154(4):906-915.e907.
- Cairns AL, Schlottmann F, Strassle PD, Di Corpo M, Patti MG. Racial and socioeconomic disparities in the surgical management and outcomes of patients with colorectal carcinoma. World J Surg. 2019;43(5):1342-1350.
- Warren Andersen S, Blot WJ, Lipworth L, Steinwandel M, Murff HJ, Zheng W. Association of race and socioeconomic status with colorectal cancer screening, colorectal cancer risk, and mortality in southern US Adults. JAMA Netw Open. 2019;2(12):e1917995.
- Bromley EG, May FP, Federer L, Spiegel BM, van Oijen MG. Explaining persistent under-use of colonoscopic cancer screening in African Americans: a systematic review. Prev Med. 2015;71:40-48.
- May FP, Yang L, Corona E, Glenn BA, Bastani R. Disparities in colorectal cancer screening in the United States before and after implementation of the Affordable Care Act. Clin Gastroenterol Hepatol. 2020;18(8):1796-1804.e1792.
- Pinsky PF, Ford M, Gamito E, et al. Enrollment of racial and ethnic minorities in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Med Assoc. 2008;100(3):291-298.
- Wallace K, DeToma A, Lewin DN, et al. Racial differences in stage IV colorectal cancer survival in younger and older patients. Clin Colorectal Cancer. 2017;16(3):178-186.
- Alese OB, Jiang R, Zakka KM, et al. Analysis of racial disparities in the treatment and outcomes of colorectal cancer in young adults. Cancer Epidemiol. 2019;63:101618.
- Ballester V, Rashtak S, Boardman L. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol. 2016;22(5):1736-1744.
- Murphy CC. Colorectal cancer in the young: does screening make sense? Curr Gastroenterol Rep. 2019;21(7):28.
- Deen KI, Silva H, Deen R, Chandrasinghe PC. Colorectal cancer in the young, many questions, few answers. World J Gastrointest Oncol. 2016;8:481-488.
Citation
MC G, JZ A. Disparities in Colorectal Cancer Outcomes Among Young Adults and African Americans in the United States. Appl Radiat Oncol. 2020;(4):10-14.
December 24, 2020