Challenges of Methotrexate Administration During Breast Radiation: A Case Report
Affiliations
- Department of Radiation Oncology, UT Southwestern Medical Center, Dallas, TX
- Department of Cardiology, UT Southwestern Medical Center, Dallas, TX
- Department of Medical Oncology, UT Southwestern Medical Center, Dallas, TX
Abstract
An increased number of patients with cancer who are receiving methotrexate for autoimmune conditions or immune checkpoint inhibitor (ICI) myocarditis are presenting to the radiation oncology department. In this article, we report a case of a patient with ICI myocarditis on methotrexate who received radiation to the chest wall and nodes. In this case, methotrexate did not increase the risk of severe acute toxicities of radiation.
Case Summary
A 29-year-old White woman presented with a palpable mass in the right breast. On examination, there was a mass at 9:00 in the right breast in addition to matted axillary nodes. Initial work-up including an ultrasound, breast MRI, and ultrasound-guided core needle biopsy revealed an infiltrating ductal carcinoma (IDC), grade 3, triple negative with Ki-67 of 75%, cT2N2M0, anatomic stage IIIA, and prognostic stage IIIC. The patient initiated neoadjuvant chemotherapy (NAC) and received 4 cycles of carboplatin, paclitaxel, and pembrolizumab, followed by doxorubicin, cyclophosphamide, and pembrolizumab for 4 cycles. After her first 4 cycles of NAC, she had a complete clinical response. This was consistent with her right breast mammogram and ultrasound, which were notable for complete response in the breast. The patient underwent right partial mastectomy and sentinel lymph node biopsy with pathological complete response, ypT0N0. The patient continued on pembrolizumab.
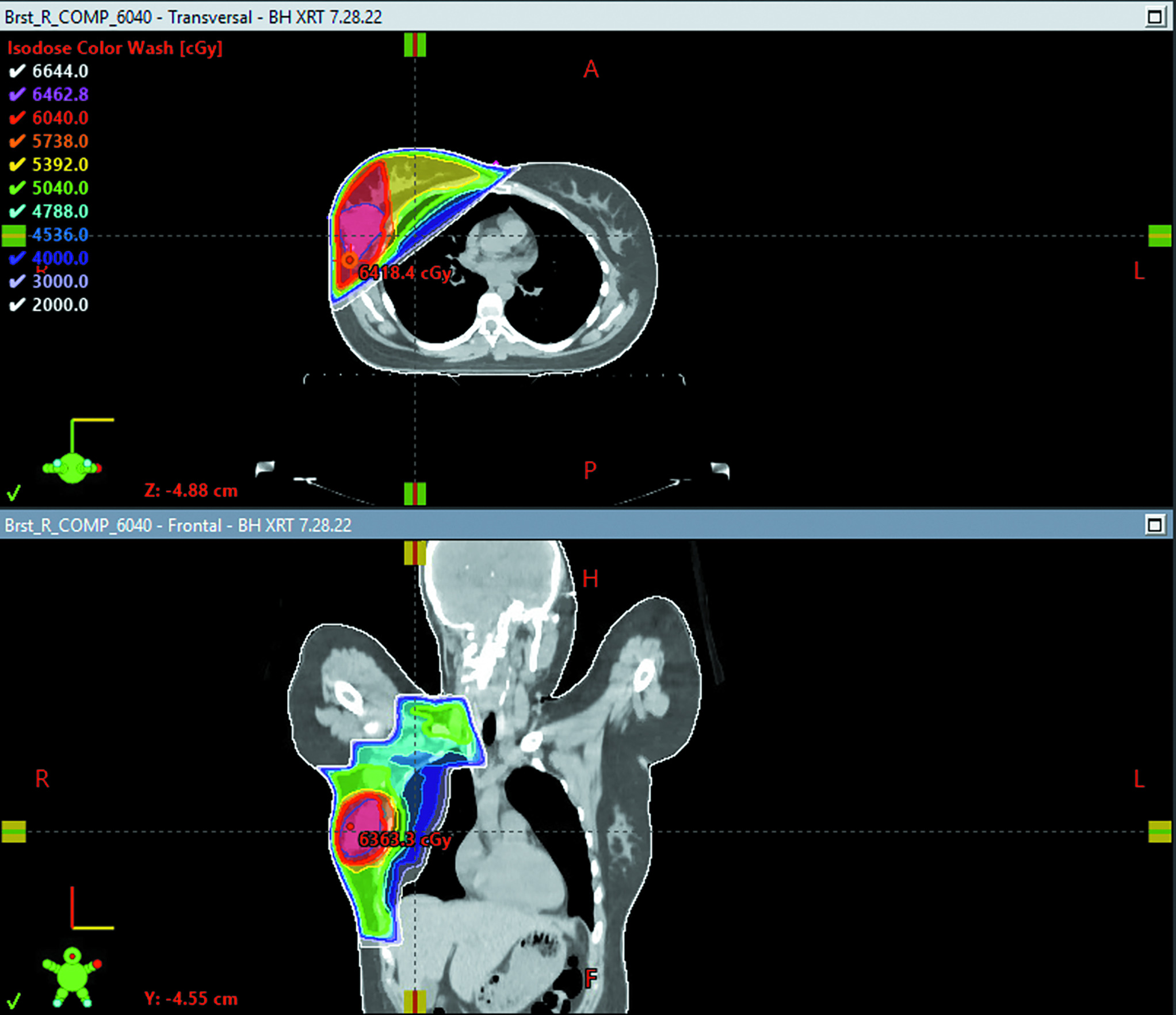
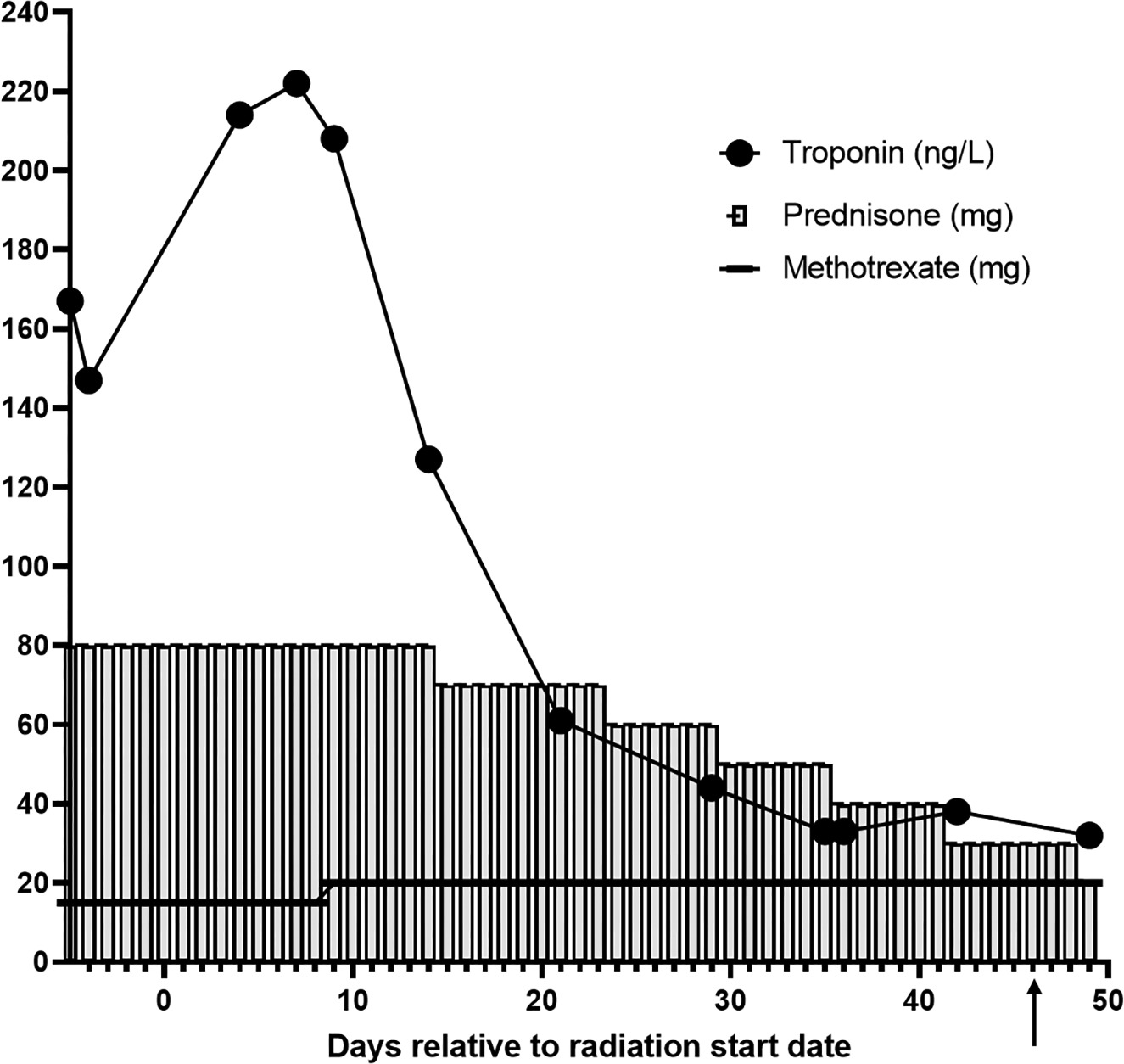
Considering the presence of bulky matted lymph nodes, young age, and triple-negative IDC, she was advised to receive comprehensive RT treatment to the right whole breast, right axilla, right internal mammary nodes, and right supraclavicular nodes. The planned dose was 50.4 Gy in 28 fractions using the deep inspiration breath hold technique ( Figures 1 , 2 ). Of note, the mean heart dose was 100.7 cGy ( Figure 2 ). The patient was simulated and within a few days prior to the start of radiation, presented to the emergency department with left-sided chest pain, nausea, and headache. Work-up revealed an elevated troponin of 404 ng/L, elevated C-reactive protein of 15.6 mg/L, and a normal electrocardiogram. Cardiac MRI was notable for normal biventricular size and systolic function in addition to late gadolinium enhancement pattern within the mid-inferolateral left ventricular wall and diffuse ventricular edema (global T2 of 68 ms) consistent with acute myocarditis per modified Lake Louise criteria.1 She was diagnosed with non-fulminant ICI myocarditis. The myocarditis was considered to be caused by both pembrolizumab (ICI) and anthracycline chemotherapy in the setting of alcohol consumption prior to chemotherapy initiation. Pembrolizumab was subsequently discontinued after she developed myocarditis. The patient was treated with 1 g of intravenous solumedrol as an inpatient, and then transitioned to prednisone 80 mg with a taper over 4 months down to 1 mg prednisone per os (PO) every other week ( Figure 3 ). At diagnosis of myocarditis, she was also started on methotrexate 15 mg PO weekly 5 days prior to the start of RT. At the start of RT, she remained on 15 mg of weekly methotrexate PO, and by day 9 of radiation, her methotrexate was increased to 20 mg weekly. She remained on 20 mg of weekly methotrexate throughout her RT treatment, and by approximately week 13 of methotrexate dosing and 5 weeks after completing radiation, her methotrexate was tapered down to 17.5 mg weekly. The start of methotrexate coincided with the beginning of RT; however, we discussed the possible toxicities of combining both treatments and decided not to delay her RT treatment and proceed with concurrent treatment, given her initial advanced stage disease and young age. Of note, this was a right-sided breast cancer, so the heart was not in proximity to the radiation field. The patient did not develop severe toxicity during radiation and concurrent oral methotrexate at her 1-month follow-up. During RT treatment, she had presented with grade 2 dermatitis in the right supraclavicular area and neck. She also developed nausea, for which she was prescribed ondansetron as needed, and mild grade 1 oral mucositis. Weekly complete blood count and troponins were checked during her treatments ( Figure 3 , Table 1 ). Her troponin levels peaked at 285 ng/L during her diagnosis of myocarditis prior to RT. During her fourth week of radiation, her troponin levels normalized and continued to stay in normal range 2 months after completion of radiation. When starting RT, her hemoglobin levels were slightly low (10.6 and 11.7 g/dl), but these normalized after the first week of RT.
Axial view of the patient’s CT-based radiation treatment plan summary. The right whole breast and regional lymph nodes (axillary, supraclavicular, internal mammary) were treated utilizing a 3D conformal technique. A dose of 5040 cGy was delivered in 28 fractions of 180 cGy each. The dose was delivered using 10 MV photons, prescribed to the planning target volume (PTV). Custom multileaf collimator blocking was used to shield uninvolved tissue. This was followed by a boost to the tumor bed delivered with 10 MV photons, utilizing a 3D conformal technique, prescribed to PTV. A boost dose of 1000 cGy was delivered in 5 fractions of 200 cGy each. Dose color wash is represented in cGy.
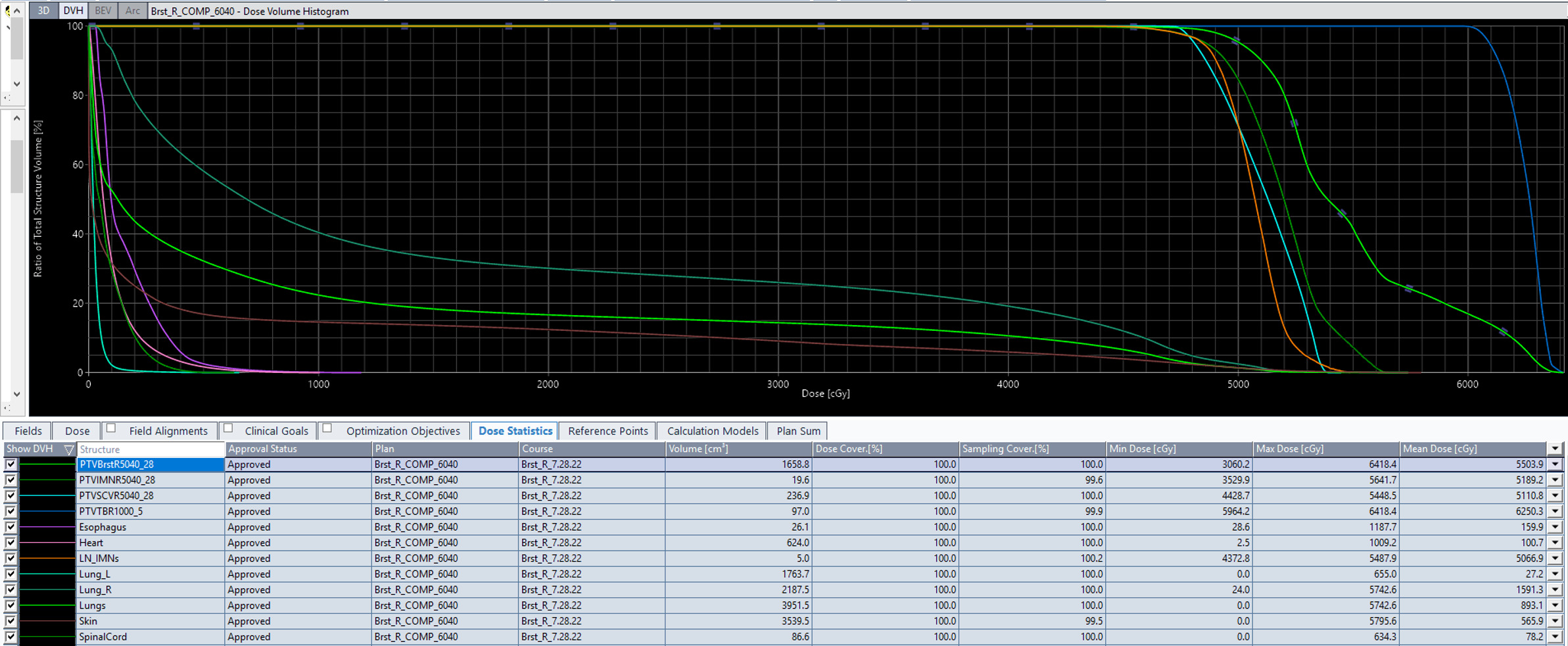
Dose volume histogram of the radiation treatment plan summary.
Trending troponin (ng/L) levels are shown before, during, and after radiation therapy, as is the correlation with prednisone (mg) and methotrexate (mg) dosing relative to the number of days before, during, and after radiation treatment. The black arrow on day 46 indicates the end of radiation treatment. The increase in the troponin level after the initation of RT is not due to the RT treatment but is likely due to the ongoing toxic effect of the alcohol.
Complete Blood Count Trends Before, During, and After Radiation Therapy (RT)
| PRE-RT | 1ST WEEK OF RT | END OF 3RD WEEK OF RT | 5TH WEEK OF RT | END OF 6TH WEEK OF RT | 5 WK POST-RT | |
|---|---|---|---|---|---|---|
| White blood cell 4.00-11.00 × 10⁹/L |
9.63 | 9.07 | 8.76 | 6.43 | 5.43 | 2.74 |
| Hemoglobin 12.0-15.0 g/dL |
10.6 | 11.7 | 12.4 | 13.3 | 13.0 | 12.5 |
| Hematocrit 34.0%-44.0% |
31.7 | 37.3 | 39.8 | 42.2 | 40.6 | 38.1 |
| Platelet 150-450 × 10(9)/L |
285 | 280 | 221 | 240 | 186 | 247 |
| Neutrophils 1.50 × 7.40 × 10(9)/L |
8.22 | 7.81 | 7.95 | 4.92 | 4.16 | |
| Lymphocytes 1.10-3.90 × 10(9)/L |
.95 | .81 | .33 | .91 | . 61 | |
| Monocytes 0.10-0.90 × 10(9)/L |
.42 | .31 | .37 | .47 | .56 | |
| Eosinophils 0.00-0.70 × 10(9)/L |
.00 | .08 | .02 | .04 | .02 | |
| Basophils 0.00-0.20 × 10(9)/L |
.00 | .02 | .01 | .03 | .02 | |
| Granulocytes 0.00-0.06 × 10(9)/L |
.01 | .04 | .08 | .06 | .06 |
Bolded numbers indicate abnormal values.
Discussion
Methotrexate is an antimetabolite, commonly used to treat rheumatoid arthritis, psoriasis, Crohn’s, and other autoimmune conditions. In addition, methotrexate is also used to treat ICI myocarditis, a condition that is more common nowadays in patients with cancer. Patients who are on methotrexate can be referred to radiation oncologists for treatment of their cancers, and the common concern is the possible toxicities of combined methotrexate with RT, which can include bone marrow suppression, mucositis, and nausea. Due to the chronicity of these medical conditions, it is not always possible to stop methotrexate during RT. To our knowledge, there are no published data on the safety of oral methotrexate during breast RT. Therefore, in this case report, we shared our experience.
With the recent results from the Keynote 522 trial, which showed an improvement in pathological complete response in patients with triple-negative breast cancer who received pembrolizumab with NAC, we expect to see more immunotherapy being used in the coming decade.2 Thus, with increased immunotherapy use, patients will most likely experience an increase in common side effects such as febrile neutropenia, anemia, as well as myocarditis, a relatively rare but serious and potentially fatal side effect.2 Though ICI-related myocarditis has a reported incidence of 0.04% to 1.14%, compared with other immune-related adverse events, ICI-related myocarditis is associated with a significantly higher mortality of 25% to 50%.3 Per the European Society of Cardiology guidelines,4 ICI myocarditis usually occurs within the first 12 weeks of ICI administration but later cases (after 20 wk) can happen. Common treatments for myocarditis include intravenous methylprednisolone at high doses (500-1000 mg daily for 3-5 d) with subsequent tapering with clinical improvements (troponin reduction by 50% within 24-72 h in addition to resolved left ventricular dysfunction, atrioventricular block, and arrhythmias). If the patient does not respond to steroids, other immunosuppressive agents such as methotrexate can also be added.
Currently, to our knowledge, there are no data examining the safety of methotrexate with concurrent RT to the breast or chest wall. However, Recchia et al reported the outcomes of a chemotherapy regimen including methotrexate with concurrent RT in axillary-node-positive patients.5 In this study, 200 patients with node-positive breast cancer received chemotherapy with adriamycin and docetaxel, followed by RT concurrent with 6 courses of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF). Two courses of dose-dense chemotherapy with ifosfamide, carboplatin, and etoposide, supported by pegfilgrastim, were administered to patients with >5 histologically confirmed axillary lymph node metastases. The radiation dose was 50 Gy in 25 fractions to the chest wall/residual breast tissue and axillary nodes (level II and III), and supraclavicular nodes in patients with >4 positive axillary lymph nodes, followed by a 10 Gy boost to the tumor bed. The methotrexate dose was 60 mg/m2 administered every 3 weeks. The most common acute toxicity for patients receiving CMF + RT was nausea and vomiting (13%), followed by mucositis (8%), diarrhea, and leukopenia (3%). Only 3% required discontinuation of treatment due to leukopenia. This study used a higher dose of methotrexate and also added other agents to the treatment but did not show severe acute toxicities. In a retrospective study by Livi et al, concurrent CMF regimen with RT was compared with RT alone and CMF alone.6 The mean RT dose was 50 Gy to the whole breast, followed by a boost of 6-16 Gy to the tumor bed. The methotrexate dose was 40 mg/m2 repeated on days 1 and 8, every 28 days for 6 cycles. There was no difference in late toxicity. However, grade 2 acute skin toxicity was higher in the concurrent arm (21% vs 11%) and RT was interrupted more in the concurrent arm (8.5% vs 4.1%). Another study by Pan et al looked at concurrent RT (40 Gy in 20 fractions to whole brain with/without spinal RT) with intrathecal methotrexate for treating leptomeningeal metastasis.7 Patients received intrathecal methotrexate at a dose of 12.5 to 15 mg weekly for 4 weeks. In addition, 20% of patients developed moderate-to-severe toxicity, and the main toxicity was leukoencephalopathy (68%), followed by bone marrow suppression (22%) and mucositis (20%). This regimen was more toxic due to the extent and location of the radiation field, in addition to intrathecal administration of methotrexate and a worse baseline condition of the patients.
Based on the above findings and a lower methotrexate dose in our patient, we decided to proceed with treatment while monitoring her potential toxicities. The patient did not develop any severe toxicity from the treatment. Further research and investigations are needed as ICI-related toxicities become more prevalent and more patients may require concurrent chemoradiation with methotrexate.
Conclusion
Based on the limited available studies involving concurrent methotrexate and RT in patients with breast cancer, it seems that the combination of both treatments does not increase the risk of severe acute toxicities; however, there might be an increase in grade 2 skin toxicity and these patients should be monitored closely until more data are accumulated. The most common side effects might be nausea, vomiting, mucositis, leukopenia, and skin toxicity, which can be monitored during treatment and addressed as needed.
References
Citation
Arbab M, MEd, Hsieh M, Zaha V, Reddy S, Rahimi A. Challenges of Methotrexate Administration During Breast Radiation: A Case Report . Appl Radiat Oncol. 2024 ;(1 ):50 - 54 .
doi:10.37549/ARO-D-23-00025
March 1, 2024