Abscopal effect of radiation therapy in monotherapy in a patient with malignant melanoma
Images
INTRODUCTION
There is increasing interest in the systemic effect that radiation therapy may provide, namely the abscopal effect—the ability of locally administered radiation therapy to trigger antitumor effects at a distance in nonirradiated metastatic lesions.1 However, the abscopal effect caused by conventional radiation therapy alone has been sparsely reported.
In this work we discuss a representative case of this effect, with the objective of adding to the minimal literature on this subject.
CASE SUMMARY
We report a case of malignant metastatic melanoma in a 93-year-old woman, who is completely independent in activities of daily living. In October 2015, the patient was diagnosed with malignant melanoma in the fifth toe of the right foot with a 2.8-mm Breslow depth. Cervical, thoracic, abdominal and pelvic computed tomography (CT) scans taken in November 2015 did not demonstrate metastatic disease.
In February 2016, lymphoscintigraphy showed drainage of the radiopharmaceutical into the right inguinal region and subsequent accumulation of the radiopharmaceutical into several ganglia of this region. The 2 most proximal ganglia of the lesion (sentinel) received a dermal marking with the aid of a surgical probe. The patient underwent amputation of the toe and excision of the sentinel node in the ipsilateral inguinal region in the same month.
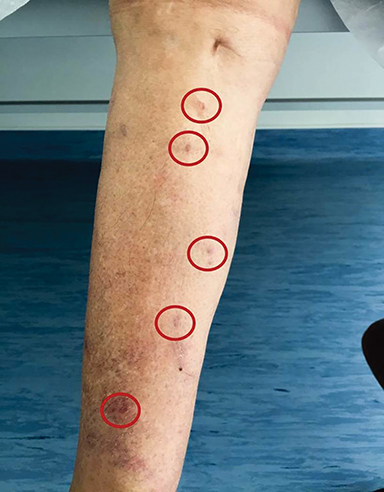
In March 2017, clinical progression of the disease was noted, with the patient noting pain in the right lower limb. Examination revealed a nodal conglomerate in the right inguinal region of about 4 cm, painful to palpation. There were also 5 cutaneous lesions (Figure 1), hard to palpation, in the region of the anterior and inner face of the right leg, with about 3 months of evolution, macroscopically compatible with in-transit metastases.
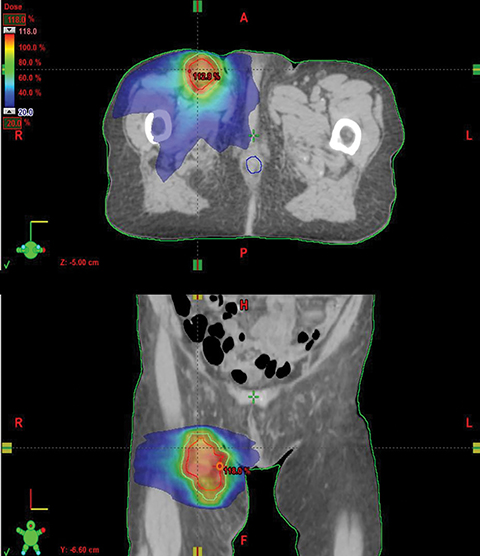
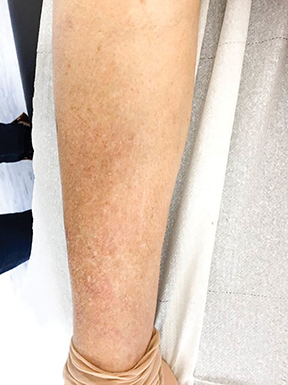
In the same month, the patient underwent hypofractionated palliative radiation therapy to the right inguinal regions with volumetric-modulated arc therapy. She was treated to a dose of 36 Gy in 3 fractions with 6 MV photons on alternate days (Figure 2). The patient did not have any other systemic therapy, such as chemotherapy or immunotherapy. One month following radiation therapy there was regression not only of the lesions within the irradiated field, which presented only as a soft patch in the inguinal region with no pain to palpation, but also in nonirradiated areas, showing only a slight alteration in epidermal pigmentation, with no significant hardened lesions on the right lower limb (Figure 3).
The patient underwent treatment without relevant side effects, and her overall status improved. The follow-up lasted a year and 2 months after the radiation therapy treatment, during which the patient did not present any treatment side effects. After then, we lost access to follow-up information since the patient moved to a different city.
DISCUSSION
Radiation therapy has been considered valuable for the control and eradication of local foci in several malignant tumors. However, there is increasing interest in the systemic effect it may produce, namely the abscopal effect.
The clinical evaluation of the abscopal effect changes the paradigm of treatment for radiation oncologists, whose main objective is to eradicate local disease, maximizing the direct death of the tumor cells, while minimizing damage to nearby normal tissue.
This concept was first reported by RH Mole in 1953,1 and has been elucidated in the recent work of several researchers such as Formenti and Demaria, who have shown that this process is probably mediated by the immune system, as ionizing radiation therapy exerts direct cytotoxic effects on tumor cells but also has the potential to enhance tumor immunogenicity by reprogramming the tumor microenvironment and eliciting antitumor T-cell responses. Radiation therapy can induce direct tumor cell death and generate inflammatory signals, such as production and release of the cytokines and chemokines into the tumor microenvironment. This causes chemoattraction and infiltration of dendritic cells—essential antigen presenting cells—and effector T cells to the tumor site. Subsequently, these properties increase the anticancer immunological response and may be responsible for the indirect anticancer effects of radiation therapy on cancers outside of the radiation field, also known as the abscopal effect.2-5
A recent review of clinical cases reporting an abscopal effect after radiation therapy treatment has shown that most reported cases have occurred in immunogenic tumors, such as renal cell carcinoma, melanoma, lymphoma and hepatocellular carcinoma.6-8
We found 2 other case reports of the abscopal effect in melanoma where the patient was only treated with radiation therapy. The first is about a 28-year-old man with a primary cutaneous melanoma lesion on his right knee.9 He also had a lymphangiogram showing abnormal inguinal, iliac and para-aortic nodes up to the level of the second lumbar vertebra. The patient was treated with fast neutrons with a dose of 14.40 Gy in 12 fractions over 35 days only to the palpable right inguinal region, with the uppermost border of the radiation field being at the level of the inferior border of the right sacroiliac joint. A repeat lymphangiogram 3 months after the radiation therapy treatment showed a remarkable regression not only on the inguinal nodes, but also on the iliac and para-aortic nodes. Nine months after treatment was started, the lymphangiogram was normal.
The second case report is about a 71-year-old man with an ulcerated malignant melanoma without lymph node involvement, which was resected.10 A year later pulmonary and mediastinal recurrence was detected. The patient refused systemic treatment. Four months later he reported pain in the right temporal region where a subcutaneous node was detected. The biopsy confirmed a melanoma metastasis. He then received local radiation therapy to the right temporal region with a dose of 30 Gy in 10 fractions, with disappearance of the palpable lesion and pain relief. During the follow-up after radiation therapy, there was a significant reduction in the size of the mediastinal nodes and disappearance of pulmonary nodules.
The total dose and fractionation of the radiation therapy treatment in these case reports are considerably different from our case, reinforcing how variable and uncharted the abscopal effect is.
This effect can be stimulated if we combine immunotherapy with radiation therapy, as reported in some published clinical cases. However, the ideal conditions and appropriate concomitant therapy are not yet known.11,12
The abscopal effect mediated by radiation therapy alone is rare and not extensively investigated. By documenting and understanding the abscopal effect of radiation therapy, we present a potential approach to help maximize local and systemic disease control in select cases.
CONCLUSION
Considering these results and the rarity of the abscopal effect with radiation therapy alone, this case report should encourage subsequent study and investigation.
Many questions remain and require clarification depending on the type of tumor and its microenvironment. Treatment timing, the total dose and fractionation of treatment (dose per fraction and number of fractions), the size of the irradiation field, and patient selection are among aspects to consider in future studies.
REFERENCES
- Mole RH. Whole body irradiation—radiobiology or medicine? Br J Radiol. 1953;26(305):234-241.
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3),862-870.
- Formenti SC, Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7),718-726.
- Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Letters. 2015;356(1),82-90.
- Ng J, Dai T. Radiation therapy and the abscopal effect: a concept comes of age. Ann Transl Med. 2016;4(6)118.
- Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40(1),25-37.
- Tsui JM, Mihalcioiu C, Cury FL. Abscopal effect in a stage IV melanoma patient who progressed on pembrolizumab. Cureus. 2018;10(2).e2238.
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10), 925-931.
- Kingsley DPE. (1975). An interesting case of possible abscopal effect in malignant melanoma. Brit J Radiol. 1975;48(574):863-866.
- de la Cruz Palomero V, Rubiales AS, García JCT, Talavera, ABF. El curioso efecto “abscopal.” Revista Clínica Española. 2014;214(3):170-171.
- Brix N, Tiefenthaller A, Anders H, Belka C, Lauber K. Abscopal, immunological effects of radiotherapy: narrowing the gap between clinical and preclinical experiences. Immunolog Rev. 2017;280(1),249-279.
- Hu ZI, McArthur HL, Ho AY. (2017). The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Cur Breast Cancer Rep. 2017;9(1),45-51.
Citation
CM S, C F, D F, G F, M L, P C. Abscopal effect of radiation therapy in monotherapy in a patient with malignant melanoma. Appl Radiat Oncol. 2019;(3):46-48.
September 4, 2019