Review ArticleLung/Thoracic Cancer
Trends in intensity-modulated radiation therapy use for limited-stage small cell lung cancer: A National Cancer Database analysi
Images


Abstract
Purpose: The standard of care for limited-stage small cell lung cancer (SCLC) is concurrent chemoradiation, which can be delivered using 3-dimensional conformal radiation therapy (3D CRT) or intensity-modulated radiation therapy (IMRT). We sought to use the National Cancer Database (NCDB) to identify predictors and trends in IMRT use for limited-stage SCLC.
Methods and Materials: We queried the NCDB from 2004-2014 for limited-stage SCLC patients that received chemo therapy and definitive doses of radiation to the chest using either 3D CRT or IMRT. Univariable and multivariable analyses were performed to identify sociodemographic, treatment, and tumor characteristics predictive of IMRT use and overall survival (OS). Propensity-adjusted Cox proportional hazard ratios for survival were used to account for indication bias.
Results: We found 9970 patients treated as above, with 59% being treated with 3D CRT and 41% being treated with IMRT. The use of IMRT increased steadily between 2004 and 2014, starting at a rate of 11% and ending at 57%. Patients with higher education and treatment at an academic center were more likely to have received IMRT, as were those receiving higher radiation dose and BID (twice daily) fractionation. IMRT use did not predict for overall survival (OS). Predictors for OS on propensity-adjusted Cox analysis were BID treatment, younger age, female gender, and private insurance.
Conclusions: The use of IMRT in limited-stage SCLC has steadily increased over the past 10 to 15 years. We expect these rates to continue to climb based on extrapolation from recommendations for non-small cell lung cancer (NSCLC).
Small cell lung cancer (SCLC) is an aggressive, high-grade neuroendocrine tumor that accounts for about 15% of all lung cancers and 20 000 to 30 000 new cases per year.1 SCLC typically presents as extensive stage, with limited stage accounting for about 30% of new diagnoses. Limited stage is classically defined as disease limited to the ipsilateral hemithorax and regional nodes that can be encompassed in a safe radiation therapy field. The standard of care treatment approach for limited-stage SCLC is chemoradiation typically using a platinum-based agent in combination with etoposide.2-5 Multiple treatment options exist in terms of radiation fractionation, including daily treatment, twice daily treatment, and occasionally a concomitant boost technique. At the time of this writing, Radiation Therapy Oncology Group (RTOG) trial 0538 remains open to help determine which scheme is most efficacious.
The technique used to deliver radiation to targets in the lung can likewise differ and varies from 3-dimensional conformal radiation therapy (3D CRT) and intensity-modulated radiation therapy (IMRT) (which use photons), to even use of protons.6,7 IMRT is used to deliver a highly conformal dose of radiation with rapid falloff to spare surrounding critical structures in the chest such as the normal lung, spinal cord, esophagus, and heart. The goal of IMRT is to help reduce treatment-related toxicity, but the technique has also been used to dose escalate. RTOG trial 0617 was a landmark non-small cell lung cancer (NSCLC) study that examined dose escalation and targeted therapy use. In that study, IMRT was utilized in about 50% of cases, and the technique was associated with less pneumonitis and a decreased heart dose, which was shown to be an important predictor for overall survival.8,9 Since the dose of radiation used with daily treatment for SCLC is similar to that of NSCLC, IMRT has been used to treat those patients as well. There are some institutional series comparing the technique in NSCLC and SCLC, potentially showing decreased toxicity.10,11 Currently, the overall utilization rate of IMRT in SCLC is unreported, although it is presumably increasing based on extrapolation of results from RTOG 0617.
In the present study, we aim to use the National Cancer Database (NCDB) to examine trends in use of IMRT in limited-stage SCLC over time, and to see if those trends impact outcome.
Methods and Materials
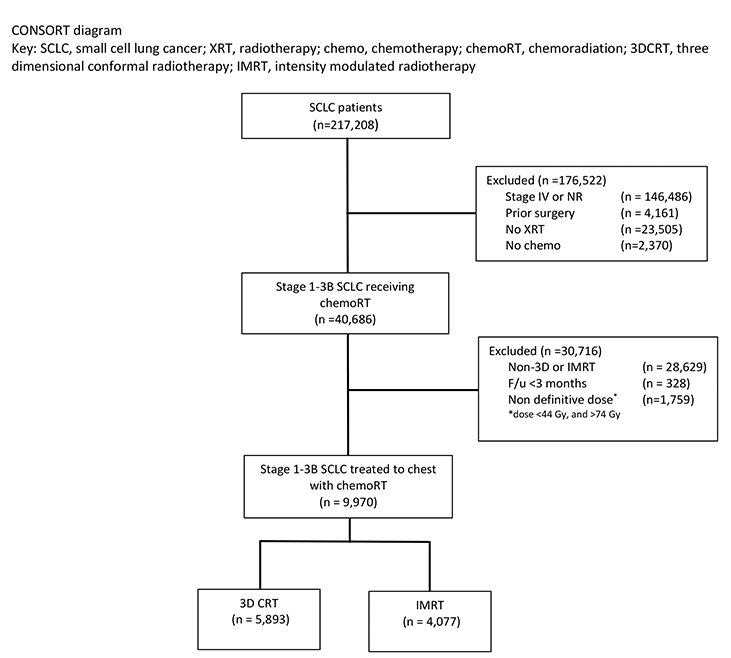
We conducted a retrospective review using de-identified data from the National Cancer Database (NCDB), which is exempt from institutional review board (IRB) oversight. The NCDB is a tumor registry maintained by the American Cancer Society and the American College of Surgeons (ACS) for more than 1500 hospitals accredited by the Commission on Cancer. The database captures up to an estimated 70% of newly diagnosed malignancies each year in the United States. We queried the database for patients with American Joint Committee on Cancer (AJCC) clinical stage 1-3B small cell lung cancer diagnosed between 2004 and 2014. Figure 1 is a Consolidated Standards of Reporting Trials (CONSORT) diagram outlining the cohort selection criteria. We excluded patients with documented stage IV disease or unrecorded stage. Patients with prior surgery, no documented radiation, or no documented chemotherapy were also excluded. We excluded patients treated with unknown radiation type or non-3D, non-IMRT techniques. To account for immortal time bias, patients were also excluded if follow-up was < 3 months, the maximum allowable time from diagnosis to start radiation therapy.12 We also used dose cutoffs of ≤ 44 Gy and > 74 Gy to define a “definitive” dose for our cohort. Patients also had to have radiation directed at the chest or thorax as coded by the NCDB, and not another anatomic site.
Race was categorized as white, African-American, or other. Comorbidity was quantified using the Charlson/Deyo comorbidity index.13 Stage was defined according to the 7th edition of the AJCC’s clinical group. Socioeconomic data in the patients’ residence census tract were provided as quartiles of the percentage of persons with less than a high school education and median household income. The facility type was assigned according to the CoC accreditation category. Locations were assigned based on data provided by the U.S. Department of Agriculture Economic Research Service. Insurance status is documented in the NCDB as it appears on the admission page. The data used in the study are derived from a de-identified NCDB file. The ACS and the CoC have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigator.
Data were analyzed using MedCalc Version 18 (Ostend, Belgium). Summary statistics are presented for discrete variables. 2 tests compared sociodemographic, treatment, and tumor characteristics between the treatment groups. Overall survival was calculated in months from time of diagnosis to date of last contact or death. Kaplan-Meier curves were used to calculate cumulative probability of survival.14 Log-rank statistics were used to test whether there was a statistically significant difference in the cumulative proportions across groups. A Cox proportional hazards model was used for multivariable survival analysis.15 Due to the large nature of the dataset, factors significant on univariable analysis were entered using a stepwise backward elimination process. Adjusted hazard ratios and 95% confidence intervals are reported, using an α level of 0.05 to indicate statistical significance.
Propensity score-matched survival analysis was used to account for indication bias due to lack of randomization between patients receiving 3D CRT and IMRT.16 Multivariable logistic regression was used to calculate a propensity score indicative of conditional probability of receiving IMRT compared to 3D CRT. The propensity model included observable variables associated with treatment selection on multivariable logistic regression. A Cox proportional hazards model was then constructed incorporating the propensity score, but also excluding factors included in the propensity score calculation to avoid overcorrection. The assumption of balance was further validated by stratifying the data into propensity score-based quintiles and confirming that the difference in propensity score mean per quintile was < 0.10.
Results
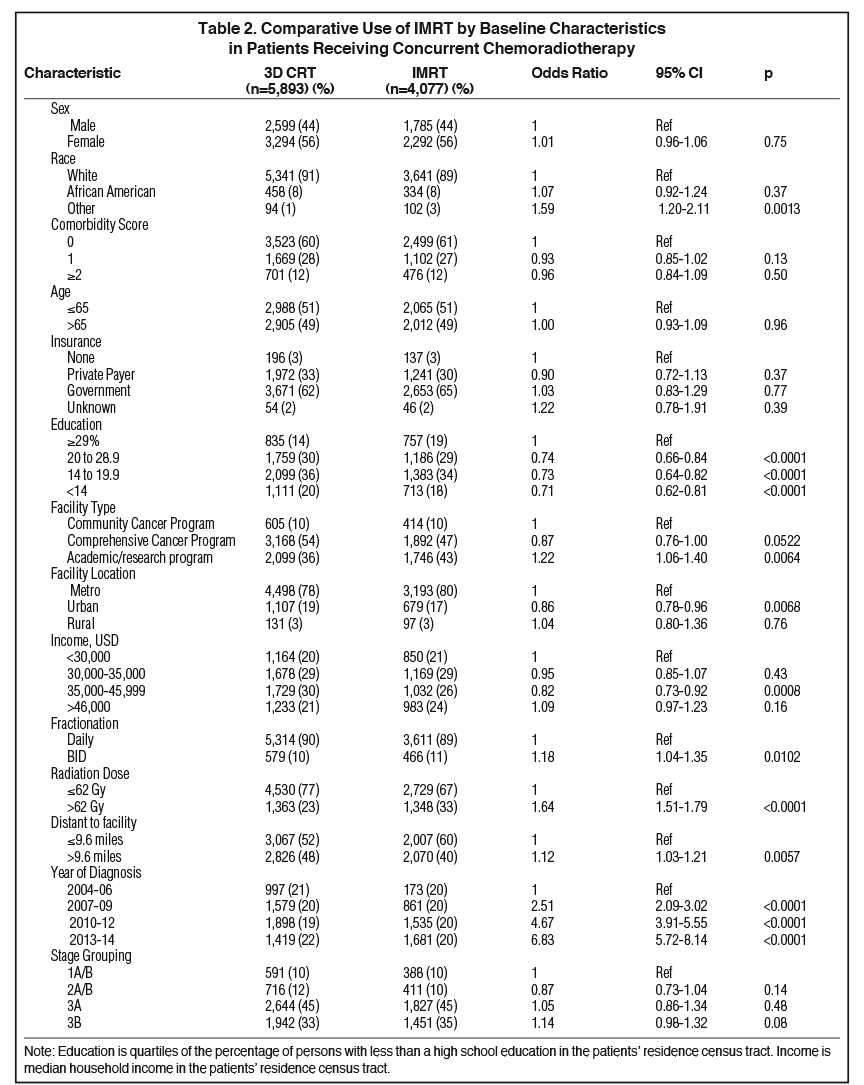
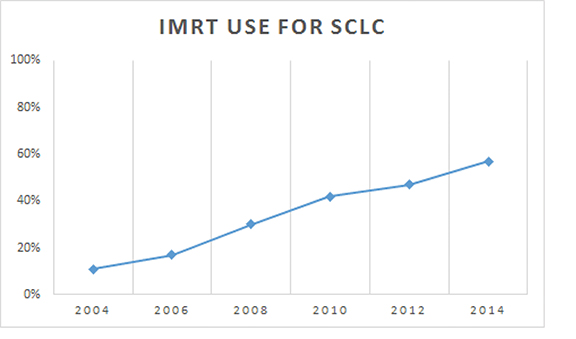
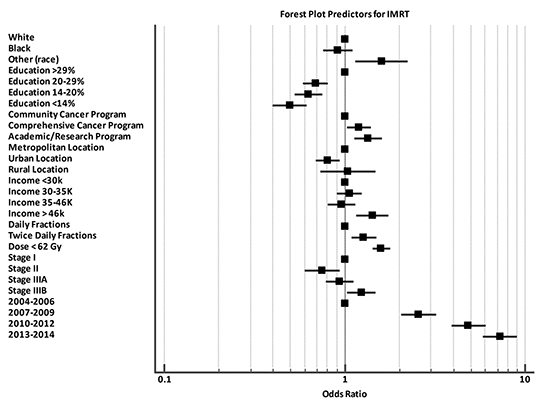
Baseline patient characteristics are outlined in Table 1. Briefly, most patients (79%) were stage 3A/B. The median age for our cohort was 65 (range: 27-90). The median radiation dose to the chest/thorax was 60 Gy (interquartile range, 54-63 Gy). Twice daily fractionation was used in 1045 cases (11%). Radiation to the chest was started at a median 42 days after diagnosis (interquartile range, 28-71 days). Chemotherapy was started at a median 21 days after diagnosis (interquartile range, 12-35 days). IMRT was utilized in 11% of cases in 2004, and steadily rose to 57% usage by 2014 (Figure 2). Overall, IMRT was used in 4,077 of 9,970 cases (40%). The odds of receiving IMRT increased with “other” race, treatment at an academic facility, BID (twice daily) fractionation, dose > 62 Gy, increased distance to facility, treatment at an academic facility, and increasing year. The likelihood of receiving IMRT decreased with urban location and with decreased education (Table 2). On multivariable regression analysis, all predictors remained significant except for distance to treatment facility. (Figure 3).
The median follow-up time was 19.1 months (range: 3 to 154 months). The median OS was 21 months. Survival at 3 years was 32% for the entire cohort. There was no statistically significant difference in survival between patients treated with IMRT or 3D CRT (median OS 21 months in each arm). On univariable analysis, predictors for increased OS included age < 65, BID treatment, lower comorbid score, increased distance to facility, type of facility, income, private insurance, race (African-American), female gender, stage, and more recent year of treatment. For multivariable Cox proportional hazards analysis, age < 65, BID treatment, lower comorbid score, increased income, private insurance type, race (African-American), female gender, lower stage, and more recent year of treatment were found to be predictors of OS (Table 3). A second multivariable Cox proportional hazards model was used including factors significant on univariable analysis plus the propensity score. The propensity score-adjusted multivariable analysis identified age < 65, BID fractionation, female gender, and nongovernment insurance as predictors for improved OS, and treatment modality remained insignificant (Table 3).
Discussion
SCLC remains a very aggressive thoracic malignancy, presenting as true limited stage only about one-third of the time.1 For those patients, despite the challenging prognosis, true “cure” remains the goal and standard of care therapy is chest radiation with concurrent chemotherapy.2 Traditionally, 3D CRT has been used for the treatment of thoracic malignancies. With the advent of IMRT, astute radiation oncologists recognized the potential advantages the technique could provide when treating lung cancer.17 Namely, those advantages involve delivery of a highly conformal dose of radiation with rapid falloff to help spare surrounding organs at risk (reduce toxicity), and perhaps even the ability to dose escalate.
The report herein appears to be the first of its kind to examine trends in IMRT use for limited-stage SCLC across the United States.
Our results show a steady increase in the utilization of IMRT in this patient population, with > 50% of patients being treated in that manner as of 2014, while only 11% received the technique in 2004. Along those lines, the odds ratio of receiving IMRT in 2013-2014 was 6.83 compared to 2004-2006. Indicators/predictors for IMRT use in this study were higher education and treatment at an academic facility, with urban patients less likely to receive IMRT. These findings likely indicate that these patients had access to more recent technology or perhaps academic radiation oncologists subspecializing in lung cancer who were more comfortable using the technique. Similarly, that urban patients were less likely to receive IMRT may indicate lack of access or other undocumented socioeconomic factors, which we can only assume played into that association.
The increase in IMRT use likely relates to extrapolation from the results of RTOG 0617, which compared 60 Gy to 74 Gy in NSCLC.8 On secondary analysis, 47% of patients in that study were treated using IMRT. Results presented in 2017 showed that patients with larger radiation therapy volumes and more advanced stage were treated with IMRT.9 Despite treating larger volumes and more advanced disease, IMRT usage allowed for a decrease in grade 3 pneumonitis and decreased heart dose, which was shown to correlate with OS. Concordantly, in our analysis, we did show a trend toward IMRT use with more advanced disease, although it was not statistically significant (p = 0.08). The authors of RTOG 0617 concluded that those results support the routine use of IMRT in NSCLC, which is frequently extrapolated to SCLC. Additionally, IMRT may provide better esophageal sparing compared with conventional techniques to mitigate this limiting toxicity of BID treatment.2
Our study did not show a survival difference with the use of IMRT, perhaps not surprisingly, as the main benefit/goal for IMRT use is to help decrease serious toxicity (data which is not included in the NCDB). We could postulate, however, that with the trend toward more advanced disease in patients receiving IMRT, a decrease in toxicity through the use of IMRT could reasonably result in an otherwise improved outcome compared to standard techniques. Our results showed that treating to a dose > 62 Gy was also a predictor for IMRT use. This indicator makes sense as treating to higher doses would make it more difficult to meet organ at risk (OAR) constraints using traditional 3D CRT. Of note, in this analysis, increasing radiation dose did not predict for improved OS. However, one could speculate that using IMRT to deliver a higher dose perhaps increased local control (not reported in NCDB), but not OS as patients with SCLC have high risk for distant disease, which is often their ultimate cause of death. A single-institution study from 2016 compared and examined outcomes in over 600 patients treated with either 3D (206 patients) or IMRT (446 patients), and showed a slight OS benefit, local control benefit, and reduced toxicity with IMRT use.11 Granted, those results were not seen in randomized data from RTOG 0617, which did not show an increase in local control or OS for NSCLC with dose escalation. We must also keep in mind that SCLC is more radiosensitive comparatively, and dose escalation in that setting will likely have diminishing returns. There is one single-institution series from MD Anderson comparing IMRT and 3D CRT for SCLC.10 Those authors reviewed outcomes in over 200 patients with limited-stage SCLC and did not show any difference in OS or disease-free survival (DFS) with IMRT. They did, however, show that IMRT patients required significantly fewer percutaneous feeding tube placements, ie, less toxicity.
We would be remiss not to mention that in our study BID fractionation was shown to have better OS compared to daily treatment (HR: 0.78, p < 0.0001). This was not the intention of the current study, nor was it taken into account when defining our cohort. In addition, this question was initially addressed with the Turrisi trial,2 and readdressed in the CONVERT trial using a higher daily dose.4 The results of the current RTOG trial will help further address that issue. Furthermore, in 2015, there was an NCDB analysis looking for differences in OS based on fractionation scheme (daily, concomitant boost, and BID), showing no significant difference among the three regimens.18
The NCDB provides a unique platform to perform well-powered retrospective analyses on a large number of patients. Nevertheless, it is subject to several limitations, namely selection bias given its retrospective, nonrandomized nature. Additionally, initial treatment response, toxicity data, salvage therapies, and disease recurrence are not included in the NCDB, all of which may affect interpretation of results. We also lack data on any follow-up radiation therapy such as prophylactic cranial irradiation (PCI), which has been shown to have an OS benefit in limited-stage SCLC and may not have been balanced between treatment arms.19 In addition, there is potential for miscoding within the data set. In that vein, we elected to exclude patients coded as being treated with photons, protons, or radiation not otherwise specified (NOS), as those codes could have been 3D CRT or IMRT and there was no reliable way to distinguish.
In summary, this study shows increased use of IMRT in limited-stage SCLC over time, with rates now eclipsing 50%. This increase in use is not unreasonable given the benefits seen when applied to our NSCLC patients. In addition, other NCDB and institutional analyses show a similar rise in IMRT use across multiple other disease sites. 20,21
Conclusions
The results of this NCDB analysis show a steady increase in the use of IMRT for the treatment of limited-stage SCLC. We expect the proportion of patients with limited-stage SCLC to continue to increase based on recent data showing reduced lung toxicity and heart dose in NSCLC.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
- Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340(4):265-271.
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327(23): 1618-1624.
- Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116-1125.
- Bogart JA, Herndon JE 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004;59(2):460-468.
- Chan C, Lang S, Rowbottom C, Guckenberger M, Faivre-Finn C, Committee IART. Intensity-modulated radiotherapy for lung cancer: current status and future developments. J Thorac Oncol. 2014;9(11):1598-1608.
- Higgins KA, O’Connell K, Liu Y, et al. National Cancer Database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97(1):128-137.
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199.
- Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol. 2017;35(1):56-62.
- Shirvani SM, Juloori A, Allen PK, et al. Comparison of 2 common radiation therapy techniques for definitive treatment of small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87(1):139-147.
- Wang J, Zhou Z, Liang J, et al. Intensity-modulated radiation therapy may improve local-regional tumor control for locally advanced non-small cell lung cancer compared with three-dimensional conformal radiation therapy. Oncologist. 2016;21(12):1530-1537.
- Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31(23):2963-2969.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-9.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481.
- Cox DR. Regression models and life- tables. J Royal Stat Soc. 1972;34(2):187-220.
- D’Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
- Cho B. Intensity-modulated radiation therapy: a review with a physics perspective. Radiat Oncol J. 2018;36(1):1-10.
- Rutter CE, Park HS, Corso CD, et al. Comparison of survival outcomes among standard radiotherapy regimens in limited-stage small cell lung cancer patients receiving concurrent chemoradiation. Lung Cancer. 2015;90(2):243-248.
- Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341(7):476-484.
- Haque W, Verma V, Butler EB, Teh BS. Utilization of intensity modulated radiation therapy for anal cancer in the United States. J Gastrointest Oncol. 2018;9(3):466-477.
- Reyngold M, Niland J, Ter Veer A, et al. Trends in intensity modulated radiation therapy use for locally advanced rectal cancer at National Comprehensive Cancer Network centers. Adv Radiat Oncol. 2018;3(1):34-41.
Citation
Wegner RE, Hasan S, Renz P, Colonias A, Turrisi AT. Trends in intensity-modulated radiation therapy use for limited-stage small cell lung cancer: A National Cancer Database analysi. Appl Rad Oncol. 2018;(4):26-33.
December 20, 2018
