Sponsored ContentGenitourinary Cancer
The Use of PSMA Targeted Therapy and Hormone Therapy in Renally Impaired Patient
Images
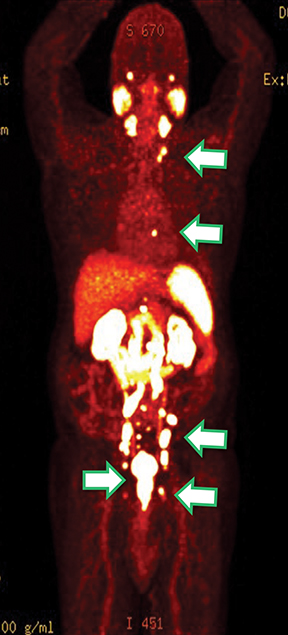
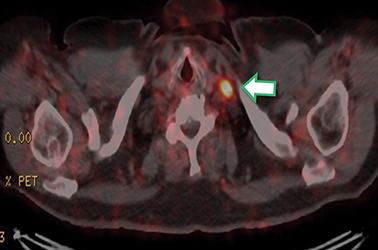
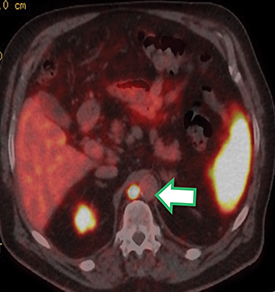
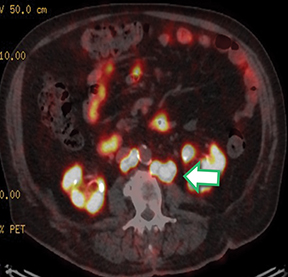


This case is brought to you by Telix.
CASE SUMMARY
An 82-year-old man presented with rising PSA of 21 ng/ml in July 2015. Prior to this time, in 2008, he received androgen deprivation therapy (ADT) and prostatic bed radiation (74 Gy). Patient was in Grade III renal failure with an eGFR of 30-40 ml/min and had previously undergone a laminectomy along with a history of osteoarthritis and lumbar stenosis.
In August 2015, the patient underwent a bone scan, which only detected degenerative changes. A CT scan found no evidence of metastatic disease. However, slightly prominent pelvic nodes of uncertain significance were noted and further evaluated with a 68-gallium (68Ga) PSMA-11 PET scan in September 2015. At the time of the PSMA PET scan, his PSA had risen to 29 ng/ml; it increased to 40 ng/ml the following month. At this time, the eGFR was 35 ml/min.
IMAGING FINDINGS
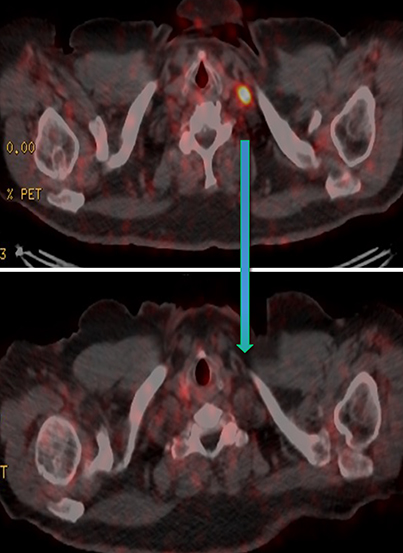
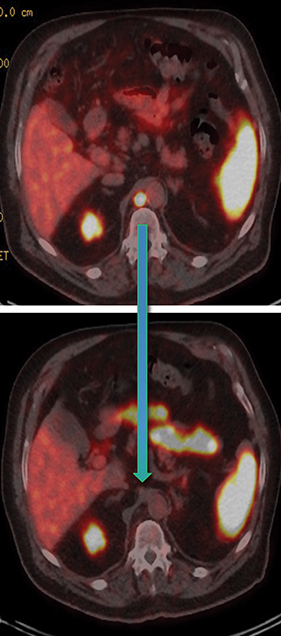
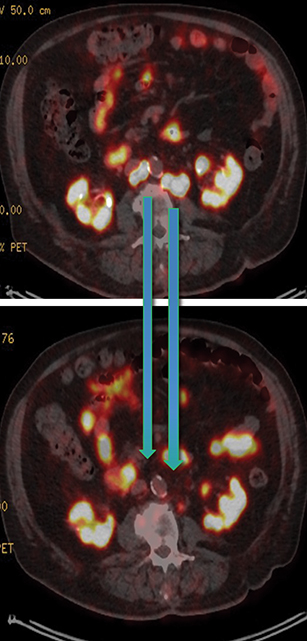
Results of the 68Ga PSMA-11 PET scan indicated prostate cancer recurrence with nodal involvement of the left supraclavicular, mediastinal, retrocrural, para-aortic and aortocaval, pelvic and inguinal regions (Figure 1).
Given the patient’s prior treatment and co-morbidities, he was offered peptide receptor radionuclide therapy (PRRT) as per institutional protocol with signed informed consent. Treatment consisted of three cycles of 177-Lutetium (177Lu) PSMA imaging and therapy (I&T): 6.77 GBq, 6.5 GBq and 4.9 GBq. Administered activities were decreased to take into account patients’ renal impairment.
Post Lu-177 PSMA treatment, the patient’s PSA fell to a nadir of 1.2 ng/ml with significant reduction in PSMA-avid lesions within the pelvis (Figures 2 and 3). Side effects of treatment included mild (grade 1) dry mouth and short-term lethargy. There was no significant change to complete blood count (CBC) or liver function tests (LFTs), and eGFR improved to 41 ml/min, likely secondary to reduction of obstructive uropathy from previous bulky adenopathy and reduction of recurrent disease at the prostatic bed.
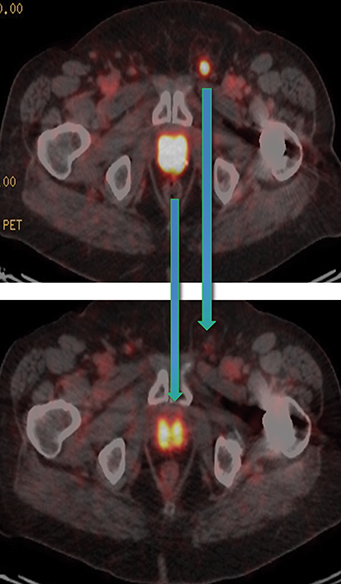
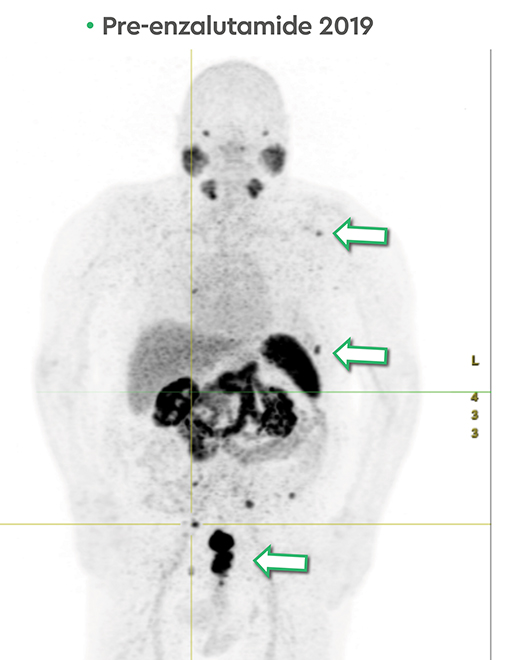
In late 2017, the PSA increased to 6.5 ng/ml with a second prostate cancer recurrence. At this time, the patient remained in renal failure (eGFR 30-40 ml/min). In early February 2018, the patient received an additional 5.98 GBq 177Lu PSMA-I&T treatment. While his PSA fell for seven months, it began to rise again, reaching 7.5 ng/ml by February 2019. The patient was initiated on intermittent low-dose enzalutamide (80 mg daily) which was well tolerated; the eGFR remained between 25-30 ml/min and other than mild anemia (hemoglobin 11.7 g/dl) his CBC, LFTs, electrolytes and lactate dehydrogenase were otherwise normal. PSA decreased to a nadir of 0.39 ng/ml by November 2019 with persistent stable biochemical profile. A PSMA PET scan prior to enzalutamide (Figure 4) revealed several small volume osseous metastases and persistent disease at the prostatic bed.
In early 2020, the patient’s PSA started rising (1.6 ng/ml) and a repeat PSMA PET scan (Figure 4) revealed resolution of most osseous PSMA avid metastases but persistence of prostatic bed disease and progressive disease in the right posterior ilium. This has been managed with an increasing dose of enzalutamide (to 120 mg daily). The latest status from May 2021 is asymptomatic from prostate cancer with stable biochemical profile and PSA of 3.5 ng/ml (PSA doubling time of greater than 12 months).
DISCUSSION
As shown with this case and as described in the literature, 177Lu PSMA therapy provides a good response in patients with nodal predominant disease1,2. 177Lu PSMA therapy is well tolerated by elderly patients and is not particularly nephrotoxic, therefore, it can be given judiciously to patients with renal impairment3,4. This treatment can be repeated safely and successfully5.
Also important, the use of novel anti-androgen therapy as an additive treatment post-radionuclide therapy may offer additional options to elderly and renal impaired patients who may not be able to tolerate chemotherapy2,6. PSMA PET may also be an aid to help delineate disease not responding to therapeutic interventions7.
CONCLUSION
PSMA PET can help monitor disease progression and treatment management, as shown in this case. The combination of therapies such as Lu-177 PSMA and enzalutamide may improve efficacy and survival.
REFERENCES
- von Eyben FE, Singh A, Zhang J, et al. 177Lu-PSMA radioligand therapy of predominant lymph node metastatic prostate cancer. Oncotarget. 2019;10(25):2451-2461. Published 2019 Mar 29. doi:10.18632/oncotarget.26789
- Emmett L, Willowson K, Violet J, Shin J, Blanksby A, Lee J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017;64(1):52-60. doi:10.1002/jmrs.227
- Yordanova A, Becker A, Eppard E, Kürpig S, Fisang C, Feldmann G, Essler M, Ahmadzadehfar H. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2017 Aug;44(9):1473-1479. doi: 10.1007/s00259-017-3681-9. Epub 2017 Mar 23. PMID: 28337529.
- Gallyamov M, Meyrick D, Barley J, Lenzo N. Renal outcomes of radioligand therapy: experience of [177Lu]lutetium-prostate-specific membrane antigen ligand therapy in metastatic castrate-resistant prostate cancer. Clin Kidney J.2019 Aug: 14;13(6):1049-1055. doi: 10.1093/ckj/sfz101. PMID: 33391748
- Aghdam RA, Amoui M, Ghodsirad M, et al. Efficacy and safety of 177Lutetium-prostate-specific membrane antigen therapy in metastatic castration-resistant prostate cancer patients: First experience in West Asia - A prospective study. World J Nucl Med. 2019;18(3):258-265. doi:10.4103/wjnm.WJNM_66_18.
- Meyrick D, Gallyamov M, Sabarimurugan S, Falzone N, Lenzo N. Real-World Data Analysis of Efficacy and Survival After Lutetium-177 Labelled PSMA Ligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Target Oncol. 2021 May;16(3):369-380. doi: 10.1007/s11523-021-00801-w. Epub 2021 Mar 9. PMID: 33687624.
- Grubmüller B, Rasul S, Baltzer P, et al. Response assessment using [68Ga]Ga-PSMA ligand PET in patients undergoing systemic therapy for metastatic castration-resistant prostate cancer. Prostate. 2020 Jan;80(1):74-82. doi:10.1002/pros.23919. Epub 2019 Oct 15. PMID: 31614001.
