Vendor UpdateInformatics & Bioinformatics
New AI Segmentation Models for Molecular Radiotherapy Get FDA Clearance
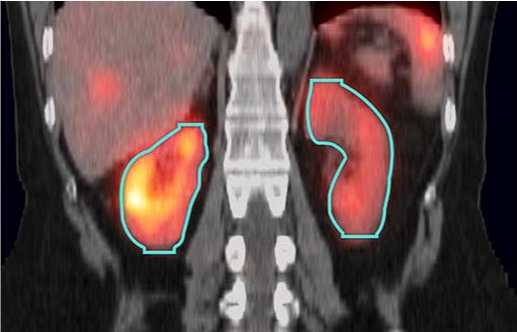 The MIM SurePlan MRT software package for Molecular Radiotherapy has received 510(k) clearance from the US Food and Drug Administration (FDA) for new AI segmentation models, which simplify the contouring process for dosimetry. AI segmentation provides significant time savings and enhanced results compared to manual and atlas-based segmentation. MIM Software’s AI segmentation models can be deployed locally or through the cloud.
The MIM SurePlan MRT software package for Molecular Radiotherapy has received 510(k) clearance from the US Food and Drug Administration (FDA) for new AI segmentation models, which simplify the contouring process for dosimetry. AI segmentation provides significant time savings and enhanced results compared to manual and atlas-based segmentation. MIM Software’s AI segmentation models can be deployed locally or through the cloud.
“We understand the challenges that are commonly faced when implementing clinical dosimetry,” said Tim Adams, Nuclear Medicine Market Director at MIM Software. “AI segmentation is one of the technologies needed to make clinical dosimetry practical. Most deployments of AI utilize cloud infrastructures and demand laborious legal agreements for the technologies to be used. However, we have made it easier for all clinics to access it by offering segmentation models in the cloud and locally.”
In addition, MIM SurePlan MRT now includes two single time point methods – the Hänscheid approach and the prior-information approach.
The Hänscheid approach uses a single SPECT/CT acquired around a carefully chosen time after each cycle of therapy to calculate the absorbed dose. The prior information approach uses time-activity curve information from multiple SPECT/CTs after the first cycle to calculate the dose for the remaining cycles, using only a single SPECT/CT at each of the remaining cycles.
MIM Software will demonstrate its new AI segmentation models in the AI Showcase at the RSNA 2021 Scientific Assembly and Annual Meeting in Chicago (November 28-December 1). MIM Software will also demonstrate MIM SurePlan MRT in booth #6327.
