Case ReportCentral Nervous System
EBRT for Treatment of Sialorrhea in Amyotrophic Lateral Sclerosis: A Case Report and Review of the Literature
Images
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease of upper and lower motor neurons resulting in weakness, debility and eventually death.1 In progressive ALS, upper motor neuron dysfunction can lead to bulbar palsy, a syndrome characterized by dysfunction of the muscles controlling speech, mastication and swallowing.2 Up to 80% of patients with ALS will develop bulbar palsy, which can result in malnutrition, dehydration, and aspiration.3 Patients suffering from bulbar palsy often experience sialorrhea, or the unintentional loss of saliva from the mouth, not secondary to increased saliva production, but rather due to an inability to swallow secretions.4 This can have a significantly negative impact on quality of life in patients whose other complications are otherwise well-managed. If conservative interventions like speech therapy, postural changes, repetitive swallowing, or biofeedback fail, medical therapy can be considered with anticholinergic medications including atropine, glycopyrrolate, amitriptyline, hycosyamine, and transdermal scopolamine.2 For refractory symptoms, more invasive local therapies can be utilized including botulinum toxin injections,5-7 external-beam radiation therapy (RT),8,15 and surgery.16-19
CASE SUMMARY
The patient was a 55-year-old woman with history of ALS diagnosed 8 years ago with initial presenting symptoms of dyspnea and sleep apnea. She developed respiratory failure requiring placement of a diaphragmatic pacemaker system shortly after diagnosis. She subsequently developed bulbar palsy symptoms 3 years ago requiring PEG-tube placement, and progressive respiratory failure requiring tracheostomy with ventilator use. Around this time, she also developed sialorrhea from copious secretions that resulted in 2 episodes of aspiration pneumonia requiring hospital admission and antibiotics. After sialorrhea symptoms were refractory to medical therapy with amitriptyline, she underwent Botox injection of the bilateral parotid and submandibular glands with relief of symptoms for 10 months. When symptoms recurred, she underwent a second Botox injection, which was effective for roughly 6 months before symptoms recurred. In the interim, her ALS progressed to the point of locked-in-syndrome with quadriplegia and loss of motor function to the lower half of her face.
With sialorrhea symptoms continuing to be bothersome and requiring frequent suctioning, the patient was referred to radiation oncology for consideration of palliative RT. After a discussion of the literature surrounding RT for ALS-related sialorrhea, the recommendation was for RT to the bilateral parotid and submandibular glands with 20 Gy in 4 fractions of 5 Gy per fraction delivered twice weekly. After considering treatment for several months with persistent sialorrhea, she returned to clinic and was consented for planning and treatment.
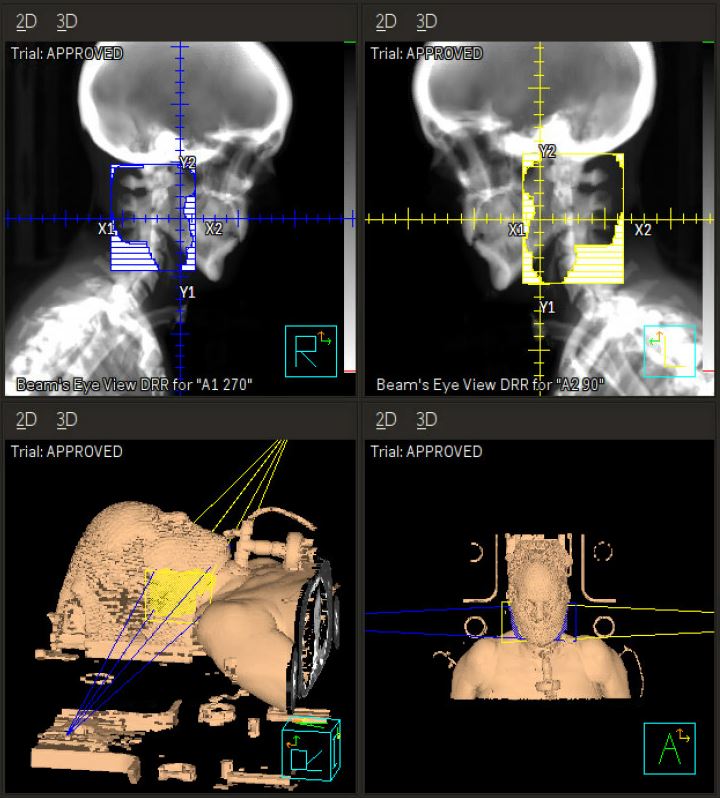
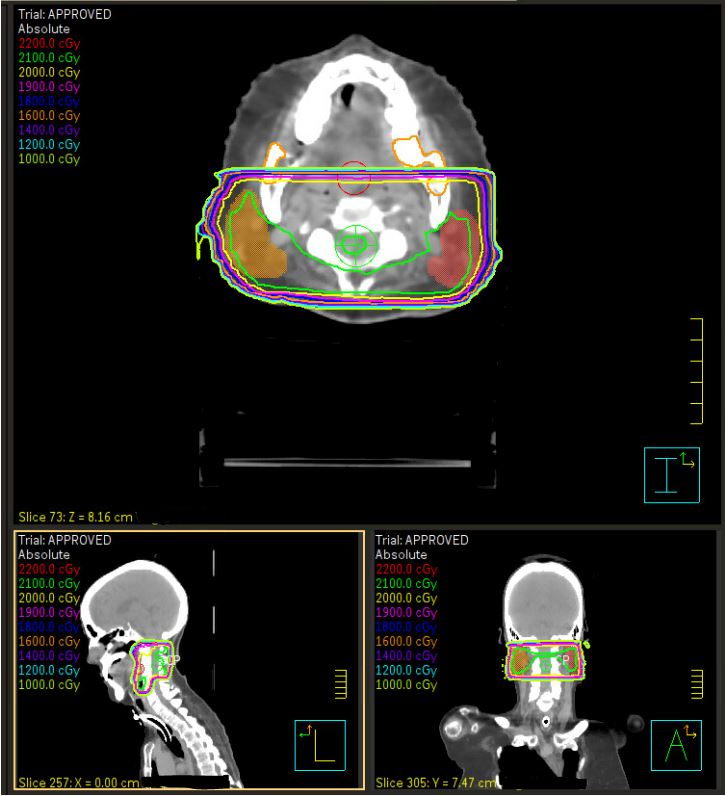
Computed tomography (CT) simulation was performed in the supine position with arms at sides (Figure 1A, B). A 3-point Aquaplast mask was utilized for motion management that would also accommodate her tracheostomy ventilator adapter. The parotid and submandibular glands were contoured bilaterally, and treatment was planned using opposed lateral technique with 6 MV photon beams prescribed to a calculation point at the 96.5% isodose line. The 4 fractions were delivered on Monday and Wednesday over 2 consecutive weeks. She tolerated treatment well, with a subjective decrease in the amount of secretions and increased thickness of saliva by the final fraction requiring less suctioning per her caregivers. The only acute adverse effects noted were mild jaw and chin discomfort after the first fraction related to the mask and trace erythema of the bilateral neck after the final fraction.
DISCUSSION
Sialorrhea due to progressive ALS is associated with decreased quality of life and increased risk of developing life-threatening aspiration pneumonia; it can also require significant intervention from caregivers with frequent suctioning.20
Radiation Therapy Outcomes
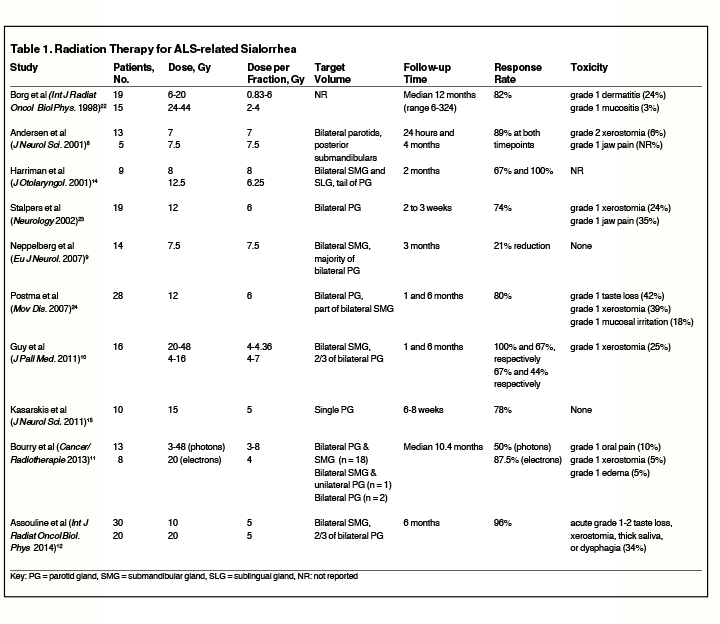
Table 1 presents a summary of studies evaluating the use of RT for sialorrhea associated with ALS. While prospective data for RT is limited for this population, Assouline et al published the largest prospective series for evaluating 50 patients with ALS-related sialorrhea treated with either 10 Gy in 2 fractions delivered on days 1 and 3, or with 20 Gy in 4 fractions delivered on days 1, 3, 8, and 10.12 Efficacy outcomes were measured using the prospectively validated 9-point Sialorrhea Scoring Scale.21 The authors report favorable results at the end of RT including improvement in all patients treated, of whom 92% experienced complete response (CR) and 8% had partial response (PR). Durable response was also seen with 71% CR and 26% PR at 6 months after RT. Both dose and fractionation schemes produced excellent responses, but the 20 Gy group had more CR and PR than the 10 Gy cohort (P = 0.02), and 8 of the 9 patients who underwent repeat RT for recurrent symptoms came from the 10 Gy arm. The dose and fractionation schedule used on the case report patient above was chosen using this study because of the prospective design and favorable outcomes. A retrospective case series examining photon RT with 15 Gy delivered in 3 fractions to unilateral parotid gland showed subjective improvement in symptoms for all 10 ALS patients included.15 The authors report 5 of the 10 patients were able to discontinue their anticholinergic medications, and 2 others were able to decrease their doses. This study suggests photon RT with 20 Gy in 4 fractions twice weekly is an effective and safe treatment for palliating ALS-related sialorrhea.
RT can be delivered with either photon or electron beams with some evidence that the treatment modality may impact efficacy. In a retrospective series by Borg et al, 82% of patients experienced satisfactory improvement in their symptoms.22 The authors reported improved response rates associated with utilization of electron-beam energy > 7 MeV when compared with orthovoltage photon beams (76% vs. 38% maintained response, P < 0.05), and with radiation fields encompassing both parotid and submandibular glands (74% vs. 33% maintained response, P < 0.01). Another study from the Netherlands by Stalpers et al delivered 12 Gy in 2 fractions to the bilateral parotid glands in 19 patients with sialorrhea, of whom 14 were treated with 250 kV photons and 5 were treated with 8 to 14 MeV electrons.23 The authors report satisfactory response to RT in 14 patients (74%) including complete response in 11 and partial response in 3 patients. However, the authors did not report a significant difference between treatment modalities. Both these studies used subjective relief of excessive salivation as the primary outcome. Two retrospective series from France compared photons to electrons for treatment of ALS-related sialorrhea.10,11 Guy et al compared efficacy and safety outcomes for photon and electron RT protocols for ALS-related sialorrhea treatment using 4-point Likert symptom improvement scores.10 Of all patients, 80% experienced improvement in symptoms at 1 month after RT, and 43% at 6 months. While both treatment modalities were equally efficacious 1 month after treatment, the authors report significantly more durable control of symptoms at 6 months in the group receiving electron RT compared to photons (P = 0.02). Bourry et al reviewed outcomes for ALS-related sialorrhea RT with 5.5-6 MV photons or 6-15 MeV electrons in 13 and 8 patients, respectively.11 The authors evaluated symptom improvement outcomes using the ALS Functional Rating Scale, reporting an overall response rate of 65% at a mean follow-up time of 7 months. The authors observed improved outcomes with electrons over photons (87.5% vs 50%, P = 0.09) and with total dose > 16 Gy compared to < 16 Gy (78.6% vs 33%, P = 0.07), although neither finding was statistically significant given the small number of patients. Together, these retrospective data suggest electron therapy is associated with improved outcomes, but that clinicians are afforded discretion regarding treatment modality chosen for RT.
Dose, Fractionation, and Target Volumes
With regard to dose and fractionation, Harriman et al examined efficacy of single-fraction vs multifraction RT for the treatment of ALS-related sialorrhea in 9 patients using a subjective questionnaire about salivary flow.14 The authors report 8 Gy in a single fraction was similarly efficacious compared with 12.5 Gy in 2 fractions, although they report long-term follow-up was limited by the shortened life expectancy in advanced ALS patients. Single-fraction RT with 12 Gy to the bilateral parotid glands was also delivered to 28 patients with sialorrhea related to Parkinsonism in a retrospective study by Postma et al.24 The authors reported efficacy in 80% of patients at 1-year follow-up when measured using the Unified Parkinson’s Disease Rating Scale questionnaire. The data, along with that by Assouline et al, suggest that longer courses can be considered for those patients with longer life-expectance; for patients with shorter life expectancy, single- or 2-fraction regimens should be considered.
While there is limited comparative data on RT for ALS-related sialorrhea, RT with 7.5 Gy in a single fraction was compared to botulinum toxin injection for ALS-related sialorrhea in a study from Norway by Neppelberg et al.9 The primary outcome was quantitative salivary flow measurement in mL/minute. While numbers were small in this trial, RT was significantly associated with improvement in salivary flow while botulinum toxin injection was not.
Target volumes generally include bilateral parotid and submandibular glands, either completely or partially covered by prescription isodose lines. The prospective trial by Assouline et al included bilateral submandibular glands and two-thirds of the bilateral parotid glands. All other retrospective series included bilateral parotid and/or submandibular glands except for one series in which authors report treatment was limited to a single parotid gland.15
Toxicity
Palliative RT for sialorrhea in ALS patients is associated with mild acute toxicity that usually is self-limited. The studies included in this review did not report any grade 3 or higher acute or late toxicities. While late toxicities were uncommon among patients treated with RT to the salivary glands, several studies reported persistent xerostomia. Borg et al reported a 13% rate of mild late toxicity, most of which was xerostomia with one case of temporomandibular joint fibrosis.22 The authors suggest late xerostomia may be ameliorated by sparing a small volume of the superior parotid gland in the treatment volume when planning RT. Andersen et al reported one case of persistent xerostomia with no other late toxicities, and several cases of acute post-RT discomfort that were relieved by administration of a lemon slice on the tongue and gentle parotid gland massage.8 Radiation doses of 8, 12.5 and 15 Gy delivered in 1, 2 and 3 fractions, respectively, were reported to have no acute or late toxicities in multiple series.14,15 In the largest series that examined 20 Gy delivered in 4 fractions, the authors report no grade 3 or 4 toxicities, and no treatment-related deaths. The rate of grade 1-2 acute toxicity was 34% and was limited to transitory taste modification, mild pain, xerostomia, salivary thickening, and swallowing difficulty. While some patients were lost to follow-up, the rates of toxicity at 1, 3 and 6 months were 8%, 15% and 5%, respectively.
CONCLUSION
RT is an effective and well-tolerated treatment option for ALS patients with bulbar palsy symptoms resulting in sialorrhea. The treatment can be delivered without significant complexity in patients with significant debility related to progressive disease, and has been shown to have low rates of significant toxicity. RT can also be considered for sialorrhea associated with other conditions, particularly Parkinson’s disease.25
REFERENCES
- Shaw PJ. Motor neurone disease. BMJ. 1999;318(7191):1118-1121.
- Banfi P, Ticozzi N, Lax A, Guidugli GA, Nicolini A, Silani V. A review of options for treating sialorrhea in amyotrophic lateral sclerosis. Respir Care. 2015;60(3):446-454.
- Oliver D. The quality of care and symptom control--the effects on the terminal phase of ALS/MND. J Neuro Sci. 1996;139 Suppl:134-136.
- Young CA, Ellis C, Johnson J, Sathasivam S, Pih N. Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis. Cochrane Database of Syst Rev. 2011(5):Cd006981.
- Costa J, Rocha ML, Ferreira J, Evangelista T, Coelho M, de Carvalho M. Botulinum toxin type-B improves sialorrhea and quality of life in bulbaronset amyotrophic lateral sclerosis. J Neurol. 2008;255(4):545-550.
- Moller E, Karlsborg M, Bardow A, Lykkeaa J, Nissen FH, Bakke M. Treatment of severe drooling with botulinum toxin in amyotrophic lateral sclerosis and Parkinson’s disease: efficacy and possible mechanisms. Acta Odontol Scand. 2011;69(3):151-157.
- Weikamp JG, Schinagl DA, Verstappen CC, Schelhaas HJ, de Swart BJ, Kalf JG. Botulinum toxin-A injections vs radiotherapy for drooling in ALS. Acta Odontol Scand. 2016;134(3):224-231.
- Andersen PM, Gronberg H, Franzen L, Funegard U. External radiation of the parotid glands significantly reduces drooling in patients with motor neurone disease with bulbar paresis. J Neurol Sci. 2001;191(1-2):111-114.
- Neppelberg E, Haugen DF, Thorsen L, Tysnes OB. Radiotherapy reduces sialorrhea in amyotrophic lateral sclerosis. Eur J Neurol. 2007;14(12): 1373-1377.
- Guy N, Bourry N, Dallel R, et al. Comparison of radiotherapy types in the treatment of sialorrhea in amyotrophic lateral sclerosis. J Pallit Med. 2011;14(4):391-395.
- Bourry N, Guy N, Achard JL, Verrelle P, Clavelou P, Lapeyre M. Salivary glands radiotherapy to reduce sialorrhea in amyotrophic lateral sclerosis: dose and energy. Cancer Radiother. 2013;17(3):191-195.
- Assouline A, Levy A, Abdelnour-Mallet M, et al. Radiation therapy for hypersalivation: a prospective study in 50 amyotrophic lateral sclerosis patients. Int J Radiat Oncol Biol Phys. 2014;88(3):589-595.
- Slade A, Stanic S. Managing excessive saliva with salivary gland irradiation in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2015;352(1-2):34-36.
- Harriman M, Morrison M, Hay J, Revonta M, Eisen A, Lentle B. Use of radiotherapy for control of sialorrhea in patients with amyotrophic lateral sclerosis. The Journal of otolaryngology. 2001;30(4):242-245.
- Kasarskis EJ, Hodskins J, St Clair WH. Unilateral parotid electron beam radiotherapy as palliative treatment for sialorrhea in amyotrophic lateral sclerosis. J Neurol Sci. 2011;308(1-2):155-157.
- Ethunandan M, Macpherson DW. Persistent drooling: treatment by bilateral submandibular duct transposition and simultaneous sublingual gland excision. Ann R Coll Surg Engl. 1998;80(4):279-282.
- Puraviappan P, Dass DB, Narayanan P. Efficacy of relocation of submandibular duct in cerebral palsy patients with drooling. Asian J Surg. 2007;30(3):209-215.
- Glynn F, O’Dwyer TP. Does the addition of sublingual gland excision to submandibular duct relocation give better overall results in drooling control? Clin Otolaryngol. 2007;32(2):103-107.
- Martin TJ, Conley SF. Long-term efficacy of intra-oral surgery for sialorrhea. Otolaryngol Head Neck Surg. 2007;137(1):54-58.
- Corcia P, Pradat PF, Salachas F, et al. Causes of death in a post-mortem series of ALS patients. Amyotrophic Lateral Scler. 2008;9(1):59-62.
- Abdelnour-Mallet M, Tezenas Du Montcel S, Cazzolli PA, et al. Validation of robust tools to measure sialorrhea in amyotrophic lateral sclerosis: a study in a large French cohort. Amyotrophic Lateral Scler Frontotemporal Degener. 2013;14(4):302-307.
- Borg M, Hirst F. The role of radiation therapy in the management of sialorrhea. Int J Radiat Oncol Biol Phys. 1998;41(5):1113-1119.
- Stalpers LJ, Moser EC. Results of radiotherapy for drooling in amyotrophic lateral sclerosis. Neurology. 2002;58(8):1308.
- Postma AG, Heesters M, van Laar T. Radiotherapy to the salivary glands as treatment of sialorrhea in patients with parkinsonism. Mov Disord. 2007;22(16):2430-2435.
- Hawkey NM, Zaorsky NG, Galloway TJ. The role of radiation therapy in the management of sialorrhea: A systematic review. Laryngoscope. 2016;126(1):80-85.
Citation
Smile TD, MD; Bauer-Nilsen K, Shah CS. EBRT for Treatment of Sialorrhea in Amyotrophic Lateral Sclerosis: A Case Report and Review of the Literature. Appl Rad Oncol. 2020;(3):38-42.
September 9, 2020
