Review ArticleGenitourinary Cancer
Daily image guidance as a noninvasive technique of rectal emptying in postprostatectomy radiation
Images

Disclosure: This research was presented in part at the ASTRO 55 Annual Meeting, Sept. 22-25, 2013, Atlanta, GA.
Abstract
Objective: To present a novel approach of rectal emptying with image-guidance in prostate bed radiation therapy.
Methods and Materials: From July 2011 to December 2012, 86 consecutively treated postprostatectomy prostate cancer patients with no evidence of metastatic disease received adjuvant/salvage radiation to 70 Gy in 35 fractions with volumetric-modulated arc radiation therapy. Prior to simulation, an enema was performed to optimize rectal anatomy for treatment planning. Daily treatment protocol consisted of a cone-beam computed tomography (CBCT) for patient setup utilizing the most caudal surgical clip and bony anatomy; if the daily rectal volume overlapped the planning target volume (PTV) by ≥ 1 cm in any axial plane on CBCT, treatment was held and the patient was asked to empty his rectum. Repeat CBCT was obtained after voiding and prior to treatment delivery. Occasionally, patients were required to undergo repeated rounds of bowel emptying and CBCT. Each day a patient was required to void, all CBCTs taken on that day were contoured over the same cranio-caudal dimensions as the primary treatment plan. The contours were transferred to the original treatment planning CT, allowing us to compare the dose-volume histogram (DVH) of the distended rectum to the actual treated rectal contours.
Results: Twenty-nine (33.7%) of 86 patients had at least 1 fraction within their course of therapy in which the rectal volume overlapped the PTV by 1 cm. An average of 2.9 (8.3%) interventions were performed among the 29 patients. Rectal volumes and average cross-sectional area (CSA) after intervention demonstrate an almost 50% reduction in volume, and CSA of the rectum with reduced rectal V70 in each case.
Conclusions: Rectal emptying when rectal filling is noted on daily CBCT prior to radiation treatment is an easy intervention to implement. This practice is associated with reduced radiation dose to the rectum and may potentially decrease rectal toxicity.
Rectal toxicity is a common complication of prostatic bed radiation, resulting in symptoms such as diarrhea and rectal urgency and, less commonly, bleeding, ulceration, incontinence and rectovesical fistula. Interventions, including endorectal balloons, have been shown to reduce rectal toxicity but are invasive and often not well-tolerated by patients.1 Rectal emptying techniques such as laxatives, daily enemas, changes in fiber intake and antiflatulent agents have been employed to produce consistent rectal anatomy.2-14 Image-guided radiation therapy (IGRT) has enabled determination of shifts in patient position to accurately target the proper internal anatomy. Often, the treatment day anatomy, and specifically the planning target volume (PTV) and organs at risk (OAR), are positioned differently from what were originally planned, prompting efforts at adaptive radiation treatment planning, which involve substantial effort and resources.15
We present a novel approach using daily IGRT, which has been successful at our institution.
Methods and Materials
From July 2011 to December 2012, 86 consecutive patients were treated at our institution with prostate bed radiation therapy to 70 Gy in 35 fractions with volumetric modulated arc radiation therapy. All patients were instructed to have a full bladder for simulation, and an enema was performed to minimize rectal volume. Institutional protocol for treating the prostate bed includes use of daily cone-beam computed tomography (CBCT) for final patient positioning using bony anatomy and the most caudal surgical clips. If the visualized rectal volume overlapped the PTV by > 1 cm in any axial plane, treatment was held and the patient was asked to empty his rectal contents, gas or feces, while continuing to drink fluids to maintain maximum bladder volume. Generally, the patient was asked to use the lavatory and attempt to walk for 20-30 minutes to assist in bowel emptying prior to proceeding. The patient was then repositioned and reimaged with CBCT to verify appropriate rectal positioning relative to the PTV. Occasionally, patients required multiple cycles of bowel emptying before adequate rectal volumes (ie, < 1 cm overlap into the PTV) was achieved.
Data acquisition
All interventions were identified by chart review. The pre-emptying and postemptying CBCTs were coregistered with rigid registration, using bony anatomy and the most apical surgical clips. In the setting of multiple interventions to achieve appropriate rectal volumes, the last CBCT was considered the postemptying CBCT. Each CBCT rectum was physician contoured over the same cranio-caudal dimensions as the primary treatment plan. Institutional rectal volumes were followed, in which the rectum contour extends 3 mm superior and inferior to the PTV. The co-registered CBCTs from each daily treatment were then fused to the planning CT in the treatment planning software, Eclipse Treatment Planning System volume 8.9 (Varian Medical Systems, Palo Alto, California), using bony anatomy and the most caudal surgical clip.
Data analysis
To minimize the effect of outliers on the dose-volume histogram (DVH) analysis, patients requiring > 3 interventions throughout their treatment course were identified and reviewed. Rectal volumes and an average cross-sectional area (CSA) were calculated on the pre- and postemptying CBCTs. Average CSA was calculated by dividing the rectal volume by the cranio-caudal length of the rectum.
Results
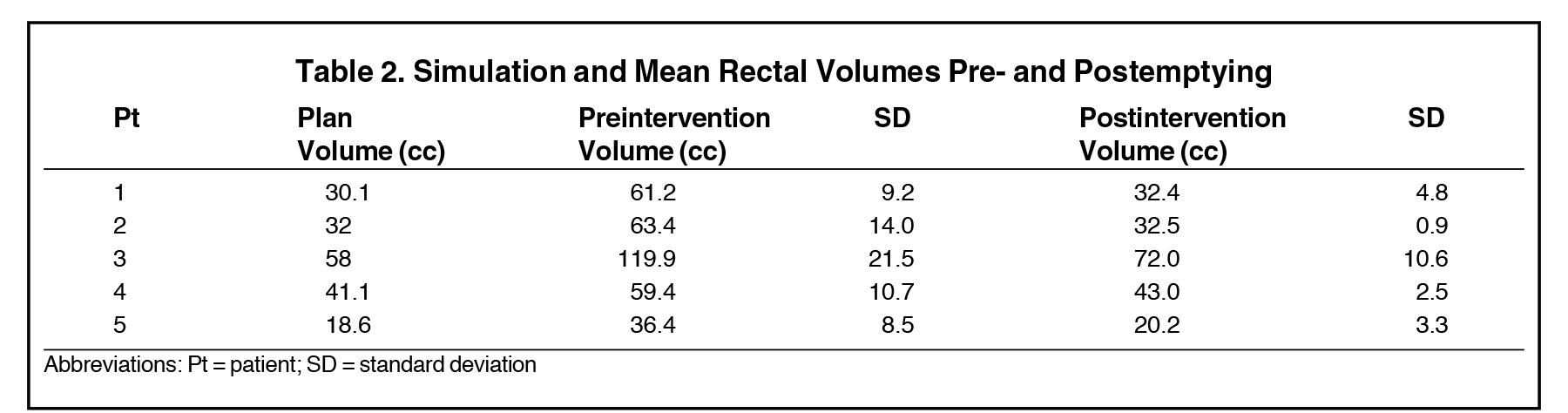
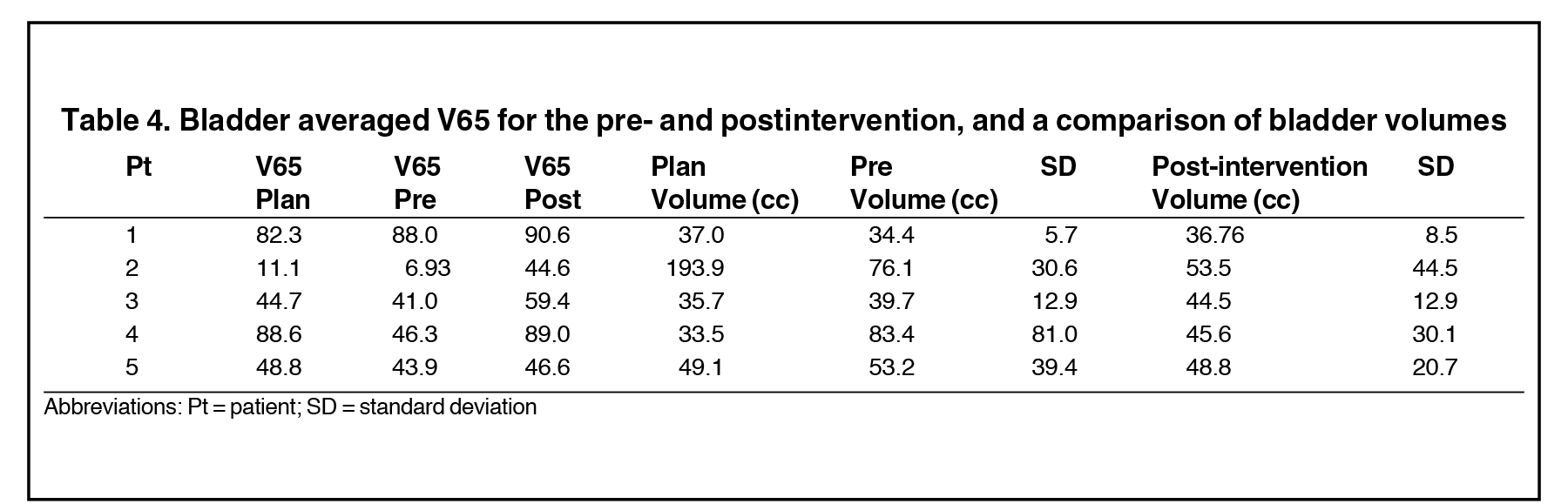
Twenty-nine of the 86 treated patients underwent at least 1 intervention of rectal voiding during their course of treatment. For each patient, this occurred an average of 2.9 (8.3%) times. Five patients had > 3 interventions; these patients were reviewed for DVH comparison and had an average of 7 interventions (range 4 to 12); the results of the rectal DVH analysis are shown in Table 1. The pre-emptying V70 for each patient is close to or > 20 percent, the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) threshold for 10% grade 3+ toxicity.16 The rectal volumes and calculated average CSA for pre- and postemptying as well as treatment planning volumes are shown in Table 2 and Table 3, and by both measures reduce volume and CSA of the rectum by almost 50%. Also, a comparison of bladder DVH and volumes are shown in Table 4.
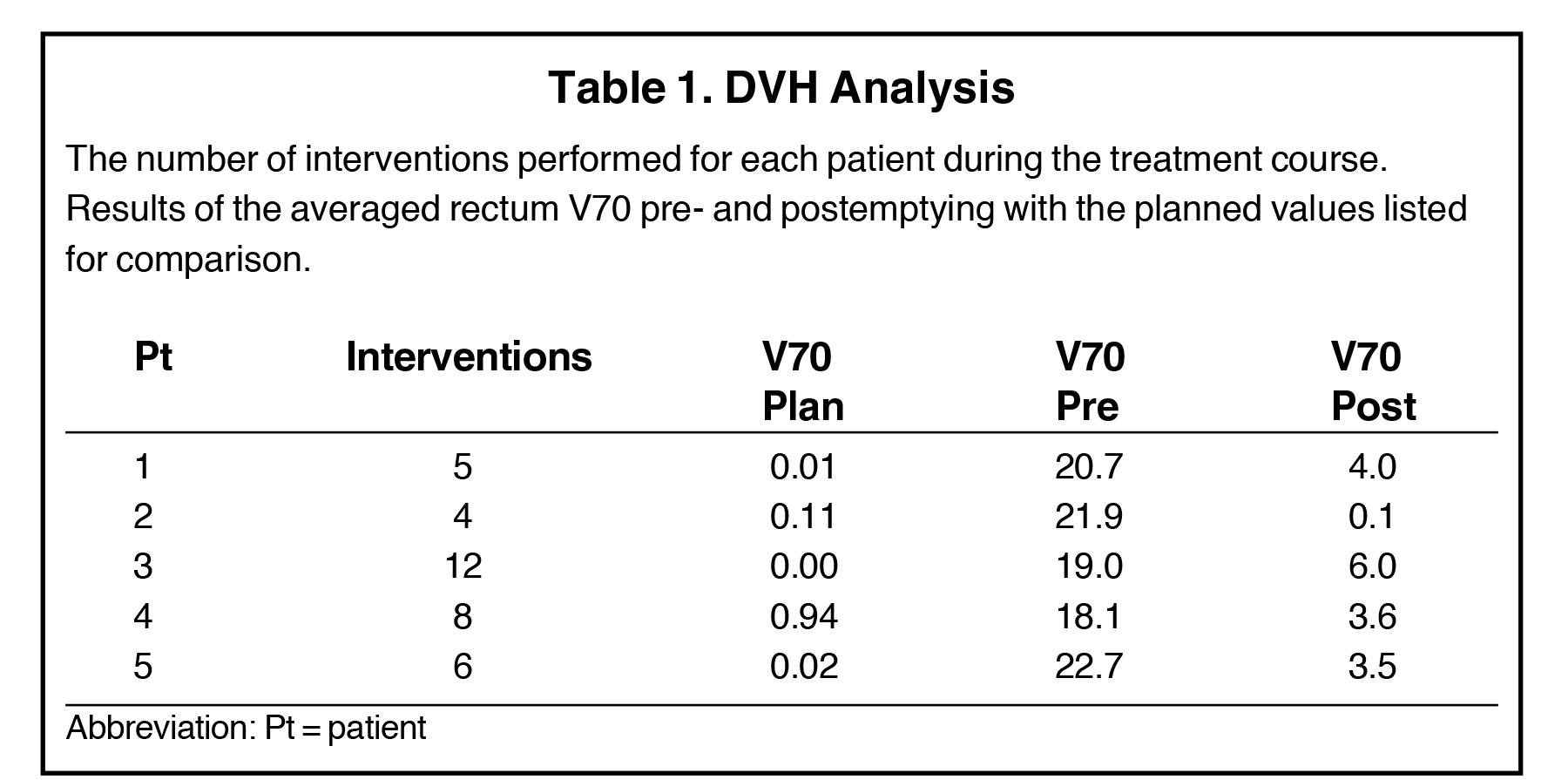
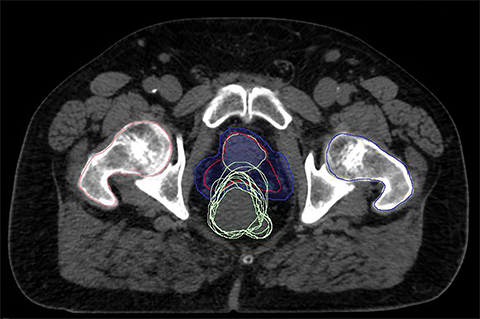
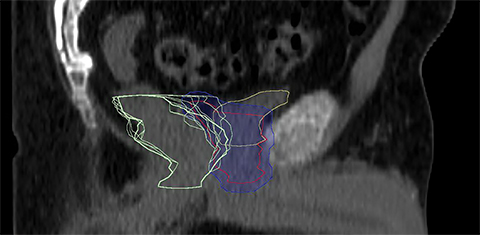
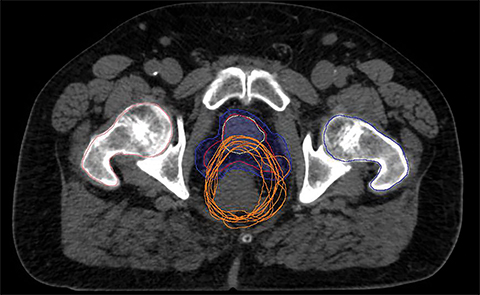
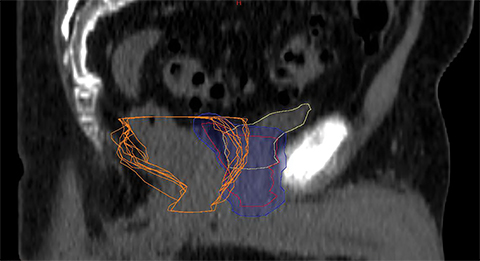
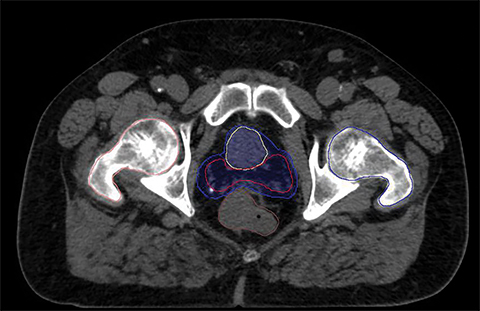
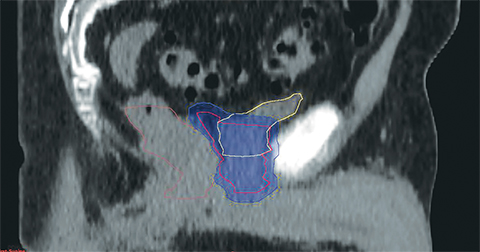
For a single patient, axial and sagittal views of the CT simulation were created for visual representation of the interventions (Figure 1). Each intervention was demonstrated with the associated contours for pre-evacuation CBCT, and postevacuation CBCT contours shown as a compilation on the treatment planning CT.
Discussion
Daily changes in rectal anatomy are a concern during prostate bed radiation therapy; attempts to overcome these changes have included invasive procedures such as daily pretreatment enemas or endorectal balloons, as well as multiple dietary and pharmacologic interventions.2-14 We proposed the use of daily CBCT to minimize rectal distortion into the PTV. If the rectum distends into the PTV > 1 cm on any axial image, asking patients to empty their rectal contents while continuing to drink fluids to maintain maximum bladder volume is a simple, noninvasive and infrequent intervention to incorporate into clinical practice.
Previous investigators have reported daily interfraction motion and filling increases the overall radiation dose to the rectum when compared to the expected dose during treatment planning,17 while others have also reported more biochemical and local failures from variations in rectal distension.18-19 Numerous prospective studies have evaluated interventions to control daily rectal distension including: diet intervention, bowel relaxants, laxatives, rectal emptying via enema, self-evacuation with finger and rectal tubes.2-14 A systematic review of numerous rectal distension interventions was unable to find a superior intervention; however, techniques employing rectal emptying before treatment were shown to be effective in decreasing rectal volumes and prostate motion.20
Although the use of various techniques are employed to limit rectal distension, our technique reveals that it may not be necessary in all patients, as only 33% of patients required a single rectal emptying intervention following daily CBCT review of the rectum. However, in patients requiring the intervention, there is a dramatic effect on reducing rectal volumes to near treatment-planning volumes with minimal effect on the bladder. This has a potential effect on target coverage as rectal distension has been reported to cause distortions in both anterior-posterior (AP) and superior-inferior (SI) motion21-25 and this has also been seen in prostate bed patients.26 With this protocol, the target is covered more reliably, potentially improving control while reducing toxicity, a classic win-win situation.
The intervention of daily CBCT with rectal voiding if necessary may also have a behavioral effect on patients. Patients who have required multiple interventions have endeavored to cooperate with timing their bowel movements before their scheduled treatments. This change in behavior as well as an increase in bowel movement frequency during radiation therapy may reduce the need for interventions in the latter half of treatment.
We also considered drawing just the rectal wall instead of the entire rectal volume. Contouring the rectal wall increases relative dose; an absolute point dose would unlikely be helpful in this scenario as well. Contouring the complete volume to include feces and gas is more comparable to routine plan review and constraints seen in QUANTEC.16 When considering the artificial increase in relative dose due to decreased contour volume, it is also imperative to consider our institution protocol of contouring the rectal volume short in the cranio-caudal dimension; a small volume magnifies the relative dose-volume. With the challenges in comparing relative dose, we also reported CSA in attempts to decrease this variability and allow comparisons to historical data.
Conclusions
We have successfully implemented in our department a noninvasive approach to managing rectal filling during prostate bed radiation therapy using daily IGRT with CBCT. If IGRT with daily CBCT is to be utilized for prostatic bed IMRT, thought should be taken to review internal anatomy for abnormalities that could be easily augmented to parallel treatment planning volumes.
References
- Smeenk RJ, van Lin EN, van Kollenburg P, et al. Endorectal balloon reduces anorectal doses in post-prostatectomy intensity-modulated radiotherapy. Radiother Oncol. 2011;101:465-470.
- Fuji H, Murayama S, Niwakawa M, et al. Changes in rectal volume and prostate localization due to placement of a rectum-emptying tube. Jpn J Radiol. 2009;27:205-212.
- Ogino I, Uemura H, Inoue T, et al. Reduction of prostate motion by removal of gas in rectum during radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:456-466.
- Padhani AR, Khoo VS, Suckling J, et al. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys. 1999;44:525-533.
- Lips IM, van Gils CH, Kotte AN, et al. A double-blind placebo-controlled randomized clinical trial with magnesium oxide to reduce intrafraction prostate motion for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:653-660.
- Oates RW, Schneider ME, Lim Joon M, et al. A randomised study of a diet intervention to maintain consistent rectal volume for patients receiving radical radiotherapy to the prostate. Acta Oncol. 2014;53:569-571.
- Nichol AM, Warde PR, Lockwood GA, et al. A cinematic magnetic resonance imaging study of milk of magnesia laxative and an antiflatulent diet to reduce intrafraction prostate motion. Int J Radiat Oncol Biol Phys. 2010;77:1072-1078.
- Smitsmans MH, Pos FJ, de Bois J, et al. The influence of a dietary protocol on cone beam ct-guided radiotherapy for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2008;71: 1279-1286.
- Stillie AL, Kron T, Fox C, et al. Rectal filling at planning does not predict stability of the prostate gland during a course of radical radiotherapy if patients with large rectal filling are re-imaged. Clin Oncol. 2009;21:760-767.
- Darud M, Giddings A, Keyes M, et al. Evaluation of a protocol to reduce rectal volume and prostate motion for external beam radiation therapy of the prostate. J Med Imag Radiat Sci. 2010;41:12-19.
- Villeirs GM, De Meerleer GO, Verstraete KL, et al. Magnetic resonance assessment of prostate localization variability in intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60:1611-1621.
- Stasi M, Munoz F, Fiorino C, et al. Emptying the rectum before treatment delivery limits the variations of rectal dose - volume parameters during 3DCRT of prostate cancer. Radiother Oncol. 2006;80:363-370.
- Fiorino C, Di Muzio N, Broggi S, et al. Evidence of limited motion of the prostate by carefully emptying the rectum as assessed by daily mvct image guidance with helical tomotherapy. Int J Radiat Oncol Biol Phys. 2008;71:611-617.
- Yahya S, Zarkar A, Southgate E, et al. Which bowel preparation is best? Comparison of a high-fibre diet leaflet, daily microenema and no preparation in prostate cancer patients treated with radical radiotherapy to assess the effect on planned target volume shifts due to rectal distension. Br J Radiol. 2013;86:20130457.
- Ost P, De Meerleer G, De Gersem W, et al. Analysis of prostate bed motion using daily cone-beam computed tomography during postprostatectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:188-194.
- Michalski JM, Gay H, Jackson A, et al. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76: S123-129.
- Fiorino C, Foppiano F, Franzone P, et al. Rectal and bladder motion during conformal radiotherapy after radical prostatectomy. Radiother Oncol. 2005;74:187-195.
- de Crevoisier R, Tucker SL, Dong L, et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning ct for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:965-973.
- Heemsbergen WD, Hoogeman MS, Witte MG, et al. Increased risk of biochemical and clinical failure for prostate patients with a large rectum at radiotherapy planning: Results from the dutch trial of 68 gy versus 78 gy. Int J Radiat Oncol Biol Phys. 2007;67:1418-1424.
- McNair HA, Wedlake L, Lips IM, et al. A systematic review: Effectiveness of rectal emptying preparation in prostate cancer patients. Pract Radiat Oncol. 2014;4:437-447.
- Langen KM Jones DT. Organ motion and its management. Int J Radiat Oncol Biol Phys. 2001;50:265-278.
- Zelefsky MJ, Crean D, Mageras GS, et al. Quantification and predictors of prostate position variability in 50 patients evaluated with multiple ct scans during conformal radiotherapy. Radiother Oncol. 1999;50:225-234.
- Roeske JC, Forman JD, Mesina CF, et al. Evaluation of changes in the size and location of the prostate, seminal vesicles, bladder, and rectum during a course of external beam radiation therapy. Int J Radiat Oncol Biol Phys. 1995;33:1321-1329.
- Schild SE, Casale HE Bellefontaine LP. Movements of the prostate due to rectal and bladder distension: Implications for radiotherapy. Med Dosim. 1993;18:13-15.
- Ten Haken RK, Forman JD, Heimburger DK, et al. Treatment planning issues related to prostate movement in response to differential filling of the rectum and bladder. Int J Radiat Oncol Biol Phys. 1991;20:1317-1324.
- Klayton T, Price R, Buyyounouski MK, et al. Prostate bed motion during intensity-modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys. 2012;84:130-136.
Citation
Elliott DA, Nabavizadeh N, Tanyi JA, Hung AY. Daily image guidance as a noninvasive technique of rectal emptying in postprostatectomy radiation. Appl Rad Oncol. 2017;(3):18-23.
September 21, 2017
