Adjuvant radiosurgery for a resected brain metastasis: A case report and literature review
Images




Case contest winner June 2015
CASE SUMMARY
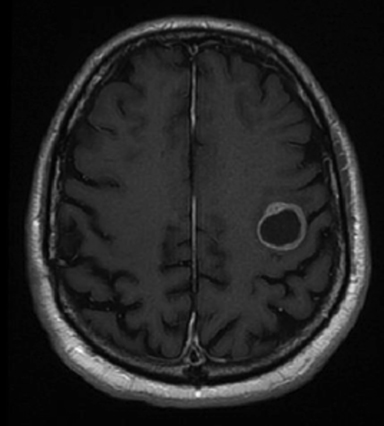
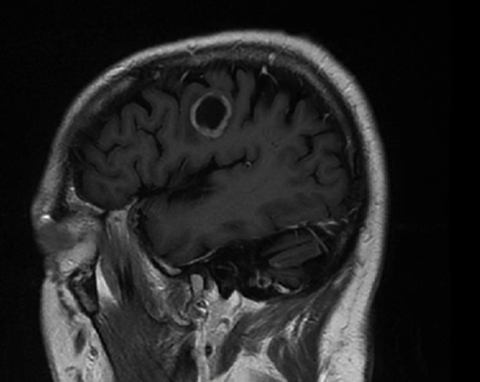
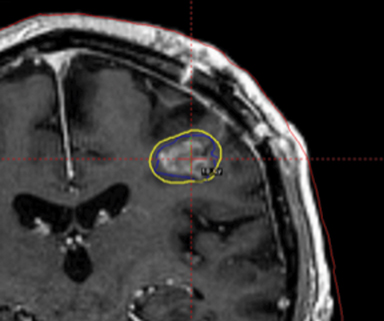
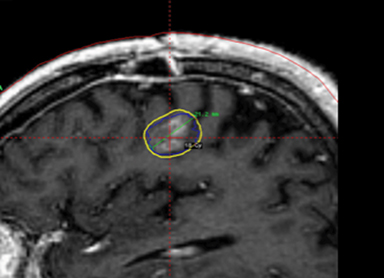
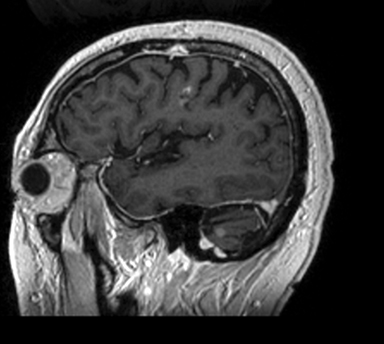
A 67-year-old Caucasian male with a remote history of prostate cancer treated with prostatectomy, salvage radiation, and anti-androgen therapy over 13 years ago presented with gradually worsening dysarthria and no other evidence of neurologic deficits. His PSA began to rise 2 years prior and was 3.6 ng/mL at the time of evaluation. MRI of the brain showed a 2.8-cm ring-enhancing lesion in the left frontal lobe (Figure 1); subsequent CT scans of the chest, abdomen, and pelvis were unremarkable. He underwent complete resection of the tumor with pathology revealing adenocarcinoma consistent with prostate origin. Adjuvant treatment with either whole-brain radiation (WBRT) vs. stereotactic radiosurgery (SRS) was discussed; the patient elected for SRS. Thirty-three days following gross total resection, he underwent Gamma Knife (Elekta, Stockholm, Sweden) radiosurgery, 18 Gy prescribed to the 51% isodose line to a target volume of 3.65 cc with a heterogeneity index of 1.961 and a conformality index of 1.504 (Figure 2).
IMAGING FINDINGS
Brain MRI showed a rounded peripherally enhancing juxtacortical mass centered along the anterior left precentral gyrus measuring 2.3 × 2.7 × 2.8 cm. Intraoperative postresection MRI demonstrated complete resection.
DIAGNOSIS
Stage IV prostate adenocarcinoma with a solitary intracranial metastasis.
DISCUSSION
Brain metastases are the most common form of brain tumor with up to 25% of patients with cancer diagnoses developing intracranial disease at some point over the course of their treatment. For several decades now, the standard of care for patients with brain metastases has been whole-brain radiation therapy (WBRT). In candidates for surgical resection, the addition of adjuvant radiation decreases local failure markedly from about 50% to 10%.1 In recent years, however, controversy has increased among oncologists about the neurocognitive effects of WBRT, with studies suggesting increased rates of dementia associated with large fraction sizes, increased probability of neurocognitive decline, decreased quality of life, and even decreased overall survival with whole-brain treatment.2-5 Other investigators have countered that neurocognitive decline is more representative of poor tumor control than radiotherapy-induced neuronal toxicity and that long-term survivors maintain neurocognitive function and equivalent overall survival.6,7 Consequentially, some argue that WBRT should be deferred in favor of the improved neurocognitive profile of SRS directed to the resection bed (adjuvant SRS). Here, we describe a case of adjuvant SRS provided to the resection cavity and explore the literature supporting the rationale and techniques applied for its delivery.
In our patient, adjuvant radiosurgery was favored given his excellent performance status, long interval between salvage prostate radiation and recurrence, and the solitary site of involvement. With this in mind, the timing and technique of his SRS needed further consideration based on existing data.
Atalar et al addressed whether waiting to deliver adjuvant SRS would allow for shrinkage of the resection cavity and, thus, minimize the radiation dose to the surrounding normal brain. They found no significant volume change up to 33 days after surgery, and concluded that there is no benefit in waiting longer than 1-2 weeks to perform cavity SRS.8
Determining the optimal margin size for patients treated with SRS has also been a subject of debate. In one of the largest series on postresection SRS, Soltys et al evaluated the local control rates associated with SRS delivered with a median marginal dose of 18.6 Gy.9 At 12 months, 80% of tumors demonstrated local control, which compared favorably to Patchell’s aforementioned study. Interestingly, among treatment factors evaluated on univariate analysis, increasing conformality and decreasing margin sizes were associated with worse local control, with authors concluding that a 2-mm margin is optimal. Another trial attempted to answer this specific question by randomizing patients to receive SRS using either 1-mm or 3-mm margins.10 Excellent 12-month local rates were detected (> 90% in both arms) with no statistically significant difference between the 2 arms; however, biopsy-proven radiation necrosis was observed more frequently in the group for which a 3-mm margin was used (p = 0.10), raising the concern that a larger margin increases the risk of radiation necrosis. Authors concluded that a 1-mm margin was preferable since the risk of radiation necrosis may be lower with no compromise in local control.
The risk of intraoperative spill of tumor at the time of resection and subsequent risk for leptomeningeal disease (LMD) when isolating radiation to a small, highly focused volume has been evaluated in previous studies and warrants extra consideration when treating with SRS. A retrospective review found that SRS was associated with an 11% risk of LMD at 12 months, with breast histology accounting for the majority of this risk (univariate HR = 2.96).11 A subsequent study compared outcomes between WBRT vs. localized radiotherapy and again found an increased risk of LMD (HR 2.45) in those treated with highly focal radiation.12
Conflicting results and physician biases complicate the interpretation of some of this data and the subsequent decision-making process about when to employ SRS vs. WBRT as adjuvant therapy. The ongoing ALLIANCE N107C trial will help determine which modality may lead to better outcomes. This question is particularly relevant in an era of heightened reluctance to contribute to neurocognitive risks, contrasted with the need to decrease medical costs, as WBRT is significantly less expensive than SRS. In this trial, patients who have undergone resection of 1 metastasis are randomized to adjuvant WBRT vs. SRS. Primary endpoints are overall survival and neurocognitive progression. SRS dose in this trial is defined by surgical cavity volume rather than size with doses ranging from 12 to 20 Gy (Table 1) delivered with 2-mm margins.
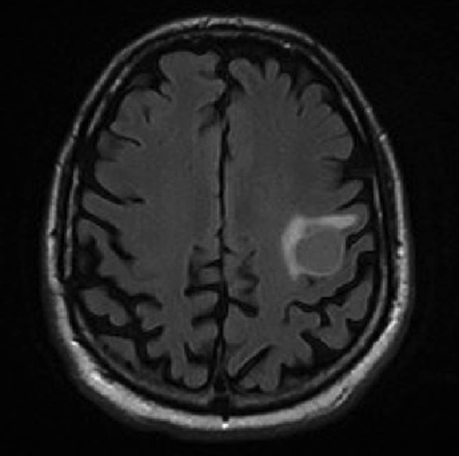
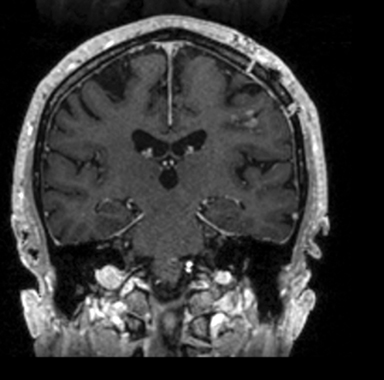
Following resection, our patient’s dysarthria initially worsened but then improved (Figure 3). He remained on androgen-deprivation therapy. Seven months after treatment, he was found to have a new single 0.7 cm intracranial metastasis in his cerebellum for which he received definitive treatment with SRS to 24 Gy. Approximately 1 year later, he developed a 3.8-cm left temporal metastasis and enrolled in an institutional protocol that permitted delivery of neoadjuvant SRS, 15 Gy in 1 session, followed by complete resection 2 days later. He continues to be monitored regularly and is doing well overall 20 months after his initial postresection radiosurgery.
CONCLUSION
This report illustrates that while there is no clear consensus on the use of SRS vs. WBRT following resection of a single brain metastasis, there is growing retrospective and phase II evidence indicating that SRS is safe and can provide effective local control in appropriately selected patients. The currently accruing N107C trial will help answer the question of whether overall survival and neurocognitive function are more affected by adjuvant WBRT vs. adjuvant SRS.
REFERENCES
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-1489.
- DeAngelis LM, Mandell LR, Thaler HT, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989;24:798-805.
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037-1044.
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
- Li J, Bentzen SM, Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64-70.
- Li J, Bentzen SM, Renschler M, et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260-1266.
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134-141.
- Atalar B, Choi CY, Harsh GR 4th, et al. Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery. 2013;72:180-185; discussion 185.
- Soltys SG, Adler JR, Lipani JD, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:187-193.
- Kirkpatrick JP, Wang Z, Sampson JH, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91:100-108.
- Atalar B, Modlin LA, Choi CY, et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2013;87:713-778.
- Hsieh J, Elson P, Otvos B, et al. Tumor progression in patients receiving adjuvant whole-brain radiotherapy vs localized radiotherapy after surgical resection of brain metastases. Neurosurgery. 2015;76:411-420.
